Human fertilization in vivo and in vitro requires the CatSper channel to initiate sperm hyperactivation
Young S, Schiffer C, Wagner A, Patz J, Potapenko A, Herrmann L, Nordhoff V, Pock T, Krallmann C, Stallmeyer B, Röpke A, Kierzek M, Biagioni C, Wang T, Haalck L, Deuster D, Hansen JN, Wachten D, Risse B, Behre HM, Schlatt S, Kliesch S, Tüttelmann F, Brenker C, Strünker T, 02.01.2024
Abstract
The infertility of many couples rests on an enigmatic dysfunction of the man’s sperm. To gain insight into the underlying pathomechanisms, we assessed the function of the sperm-specific multisubunit CatSper-channel complex in the sperm of almost 2,300 men undergoing a fertility workup, using a simple motility-based test. We identified a group of men with normal semen parameters but defective CatSper function. These men or couples failed to conceive naturally and upon medically assisted reproduction via intrauterine insemination and in vitro fertilization. Intracytoplasmic sperm injection (ICSI) was, ultimately, required to conceive a child. We revealed that the defective CatSper function was caused by variations in CATSPER genes. Moreover, we unveiled that CatSper-deficient human sperm were unable to undergo hyperactive motility and, therefore, failed to penetrate the egg coat. Thus, our study provides the experimental evidence that sperm hyperactivation is required for human fertilization, explaining the infertility of CatSper-deficient men and the need of ICSI for medically assisted reproduction. Finally, our study also revealed that defective CatSper function and ensuing failure to hyperactivate represents the most common cause of unexplained male infertility known thus far and that this sperm channelopathy can readily be diagnosed, enabling future evidence-based treatment of affected couples.
YOUNG, Samuel, et al. Human fertilization in vivo and in vitro requires the CatSper channel to initiate sperm hyperactivation. The Journal of Clinical Investigation, 2024, 134. Jg., Nr. 1; DOI: https://doi.org/10.1172/JCI173564
Publication: https://doi.org/10.1172/JCI173564 Repository: https://www.jci.org/articles/view/173564/sd/6
 Disclaimer
Disclaimer
The publication Human fertilization in vivo and in vitro requires the CatSper channel to initiate sperm hyperactivation by Young S, Schiffer C, Wagner A, Patz J, Potapenko A, Herrmann L, Nordhoff V, Pock T, Krallmann C, Stallmeyer B, Röpke A, Kierzek M, Biagioni C, Wang T, Haalck L, Deuster D, Hansen JN, Wachten D, Risse B, Behre HM, Schlatt S, Kliesch S, Tüttelmann F, Brenker C, Strünker T is published under an open access license: https://creativecommons.org/licenses/by-nc-nd/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: The CatSper-Activity-Test identifies patients with defective CatSper function
Other: Functional Study
Species
| Species |
|---|
| Human |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:0000407: Sensorineural hearing impairment | 4 | A type of hearing impairment in one or both ears related to an abnormal functionality of the cochlear nerve. |
| HP:other: Other | 3 | normozoospermic (hyperactivated motility either abolished, unaffected, or not assessed) |
| HP:other: Other | 7 | (oligo)asthenoteratozoospermic, ejaculate contained only few, poorly motile and morphological abnormal sperm |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001230: semen | adult | A viscid whitish fluid of the male reproductive tract consisting of spermatozoa suspended in secretions of accessory glands. | Human | 2286 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm | A mature male germ cell that develops from a spermatid. | Human |
Images
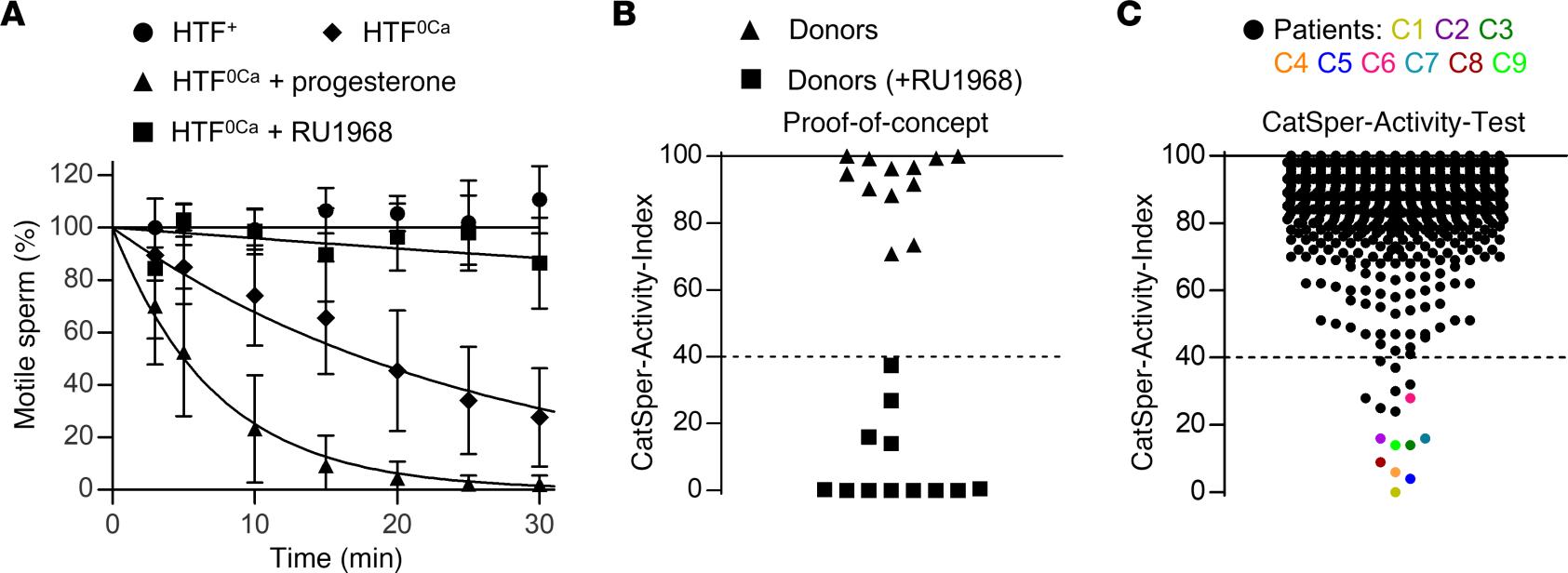
Figure 1: Development of a motility-based test to assess the activity of CatSper in human sperm.
(A) Changes in the fraction of motile sperm (mean ± SD) upon dilution of semen samples from donors in Ca2+-free HTF (HTF0Ca) (diamonds; n = 9), HTF0Ca containing progesterone (10 μM) (triangles; n = 9), or HTF0Ca containing the CatSper-inhibitor RU1968 (15 μM) (squares; n = 6), relative to the fraction of motile sperm determined upon dilution of the respective semen sample in control HTF+ (circles, n = 9) at t = 0 (set to 100%). An exponential decay curve was fitted to the change in the fraction of motile cells averaged over all replicates. (B) CatSper-Activity-Indices (CAI) determined 15 minutes after dilution of semen samples from donors (n = 12) in HTF+ (Buffer A) and HTF0Ca containing progesterone (triangles) (Buffer B) or Buffer B also containing RU1968 (15 μM) (squares). (C) CAI values from semen samples of men undergoing semen analysis (n = 2,286); the dotted line indicates the CAI threshold, i.e., values above and below were considered indicative of normal and defective CatSper function, respectively. Patients with confirmed loss or impaired CatSper function (see Figure 2) are labeled C1–C9 and indicated with a color-coded circle.
Licensed under: https://creativecommons.org/licenses/by-nc-nd/4.0/
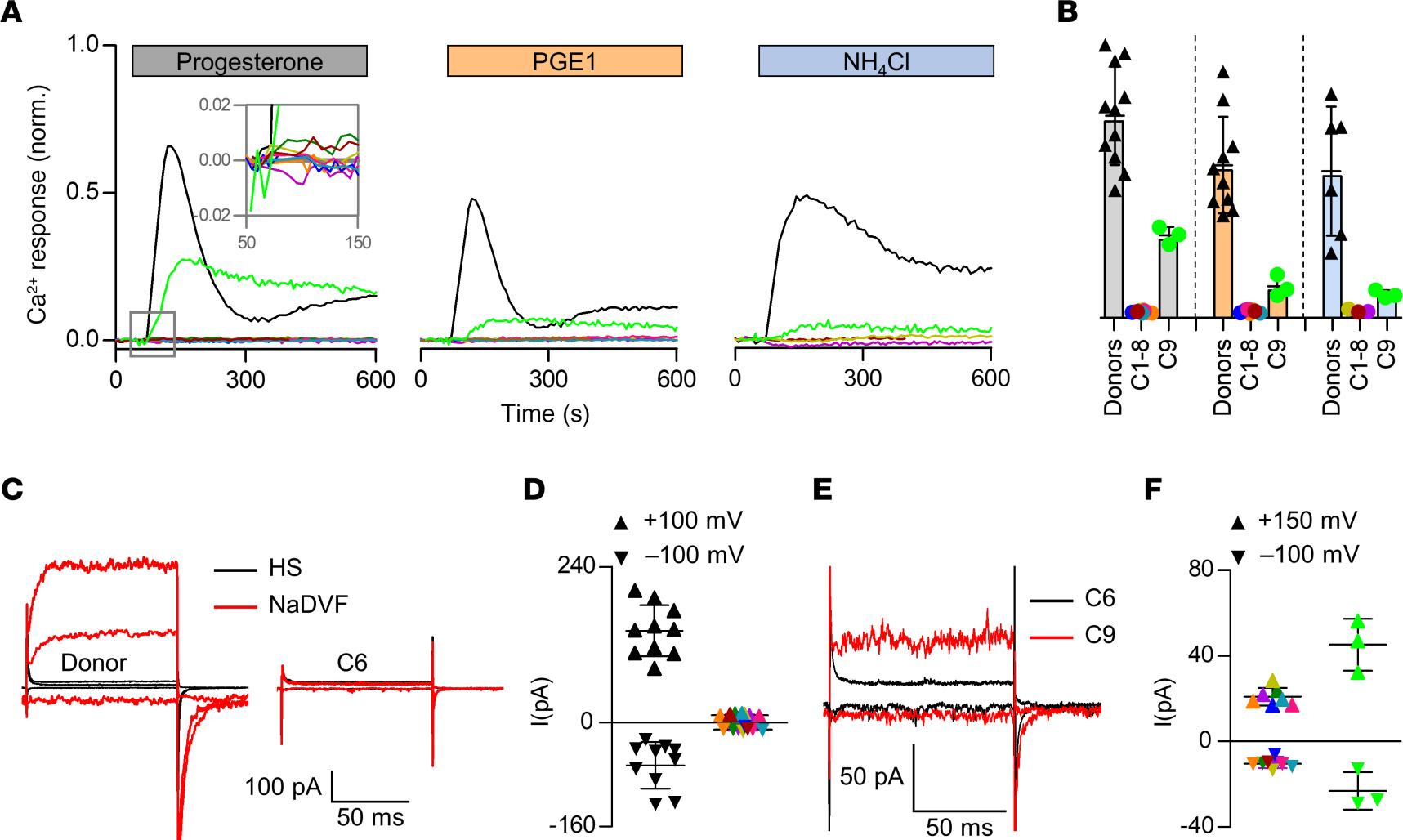
Figure 2: Ca2+ signals and membrane currents in sperm from patients with impaired or loss of CatSper function.
(A) Representative Ca2+ signals in sperm from donors and patients C1–C9 (color coded) evoked by progesterone (3 μM), PGE1 (3 μM), or NH4Cl (30 mM) relative to the maximal signal amplitude evoked by ionomycin (3 μM) (set to 1). (B) Mean (± SD) maximal signal amplitude evoked by progesterone (gray; donors n = 11, C1-C8 n = 1, C9 n = 3), PGE1 (orange; donors n = 10, C1,2,4-8 n = 1, C9 n = 3), or NH4Cl (blue; donors n = 6, C1,2,8 n = 1, C9 n = 3) relative to that evoked by ionomycin (set to 1). (C) Representative whole-cell currents recorded from a sperm cell of a donor and patient C6 in extracellular solution containing Mg2+ and Ca2+ (HS) and in Na+-based divalent-free solution (NaDVF), evoked by stepping the membrane voltage to –100mV, +100 mV, and +150 mV from a holding potential of –80 mV. (D) Steady-state current amplitudes (NaDVF) at +100 mV and –100 mV in sperm from donors (black, n = 10) and patients C1–C8 (color coded, n = 1). (E) Representative whole-cell currents recorded from patients C6 (black) and C9 (red) in NaDVF, evoked by stepping the membrane voltage from to –100mV, +100 mV, and +150 mV from a holding potential of –80 mV. (F) Steady-state current amplitudes at +150 mV and –100 mV in NaDVF in sperm from patients C1–C7 and C1–C8, respectively, (color coded; n = 1) as shown in C (consider the scales of Y-axes) compared to patient C9 (light green, n = 3). Data on Ca2+ responses and membrane currents in sperm from patients C1–C5 comprise data from ref. 44, reporting on these patients for the first time, combined with data from additional experiments.
Licensed under: https://creativecommons.org/licenses/by-nc-nd/4.0/
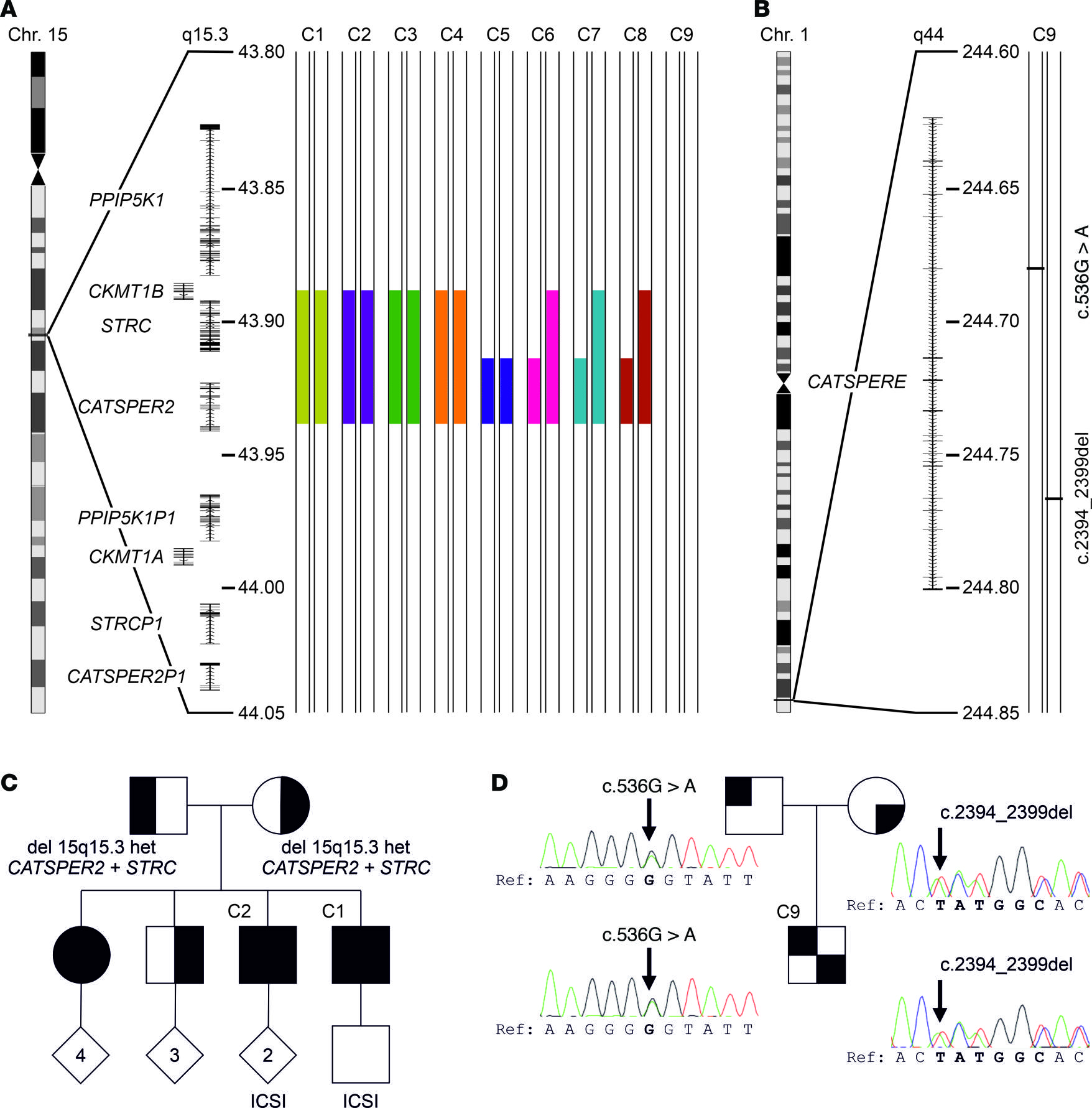
Figure 3: Genetic aberrations identified in patients with impaired or loss of CatSper function.
(A) Schematic depiction of chromosome 15, magnified region q15.3 including genes and the identified deletions (filled color-coded bars) in patients C1–C8, but not patient C9. All positions according to hg19/GRCh37. (B) Schematic depiction of chromosome 1, magnified region q44 and compound-heterozygous variants (see panel D) (c.536G>A and c.2394_2399del) of CATSPERE (NM_001130957.2) identified in patient C9. (C) Family pedigree of patients C1 and C2. Their sister is also homozygous for the deletion at 15q15.3, whereas their mother, father, and third brother are heterozygous carriers. (D) Family pedigree of patient C9, demonstrating that the father and mother are carriers of the missense variant (c.536G>A) and in-frame deletion (c.2394_2399del), respectively. Of note, in ref. 44, we previously showed array-CGH data from patients C1–C5, reporting on the deletion of CATSPER2 in these patients for the first time.
Licensed under: https://creativecommons.org/licenses/by-nc-nd/4.0/
Data set 2: Deletion of CATSPER2 in patient C9
Exome: Whole Exome Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0000553: peripheral blood | adult | Blood circulating throughout the body. | Human | 1 |
