Does the FSHB c.‐211G>T polymorphism impact Sertoli cell number and the spermatogenic potential in infertile patients?
Maria Schubert, Sophie Kaldewey, Lina Pérez Lanuza, Henrike Krenz, Martin Dugas, Sven Berres, Sabine Kliesch, Joachim Wistuba, Jörg Gromoll, 28.08.2020
Abstract
BackgroundA genetic variant within the FSHB gene can deviate FSH action on spermatogenesis. The c.‐211G>T FSHB single nucleotide polymorphism impacts FSHB transcription and biosynthesis due to interference with the LHX3 transcription factor binding. This SNP was previously shown to be strongly associated with lowered testicular volume, reduced sperm counts, and decreased FSH levels in patients carrying one or two T‐alleles.ObjectiveTo determine the impact of the SNP FSHB c.‐211G>T on Sertoli cell (SC) number, Sertoli cell workload (SCWL) and thereby spermatogenic potential.Material and methodsTesticular biopsies of 31 azoospermic, homozygous T patients (26 non‐obstructive azoospermia (NOA), and five obstructive azoospermia (OA)) were matched to patients with GG genotype. Marker proteins for SC (SOX9), spermatogonia (MAGE A4), and round spermatids (CREM) were used for semi‐automatical quantification by immunofluorescence. SCWL (number of germ cells served by one SC) was determined and an unbiased clustering on the patient groups performed.ResultsQuantification of SC number in NOA patients did not yield significant differences when stratified by FSHB genotype. SC numbers are also not significantly different between FSHB genotypes for the OA patient group and between NOA and OA groups. SCWL in the NOA patient cohort is significantly reduced when compared to the OA control patients; however, in neither group an effect of the genotype could be observed. The cluster analysis of the whole study cohort yielded two groups only, namely NOA and OA, and no clustering according to the FSHB genotype.Discussion and conclusionThe FSHB c.‐211G>T polymorphism does not affect SC numbers or SCWL, thereby in principle maintaining the spermatogenic potential. The previously observed clinical phenotype for the FSHB genotype might therefore be caused by a hypo‐stimulated spermatogenesis and not due to a decreased SC number.
SCHUBERT, Maria, et al. Does the FSHB c.‐211G> T polymorphism impact Sertoli cell number and the spermatogenic potential in infertile patients?. Andrology, 2020, 8. Jg., Nr. 5, S. 1030-1037.
Publication: https://doi.org/10.1111/andr.12777
 Disclaimer
Disclaimer
The publication Does the FSHB c.‐211G>T polymorphism impact Sertoli cell number and the spermatogenic potential in infertile patients? by Maria Schubert, Sophie Kaldewey, Lina Pérez Lanuza, Henrike Krenz, Martin Dugas, Sven Berres, Sabine Kliesch, Joachim Wistuba, Jörg Gromoll is published under an open access license: https://creativecommons.org/licenses/by/4.0/. Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Reproductive parameters in the NOA/OA patient cohorts
Proteome: Immunohistochemistry
Species
| Species |
|---|
| Human |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:0000027: Azoospermia | 62 | The 31 TT patients were matched to 31 patients (26 NOA/5 OA) (criteria for matching: age, history of maldescensus testis, varicocoele, nicotine abuse, sperm counts) carrying the FSHB GG genotype |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | Human |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000216: Sertoli cell | Marker proteins [...]were used for semi‐automatical quantification by immunofluorescence. SCWL (number of germ cells served by one SC) was determined and an unbiased clustering on the patient groups performed. | Human | |||
| CL_0000018: spermatid | Human | ||||
| CL_0000020: spermatogonium | Human |
Images
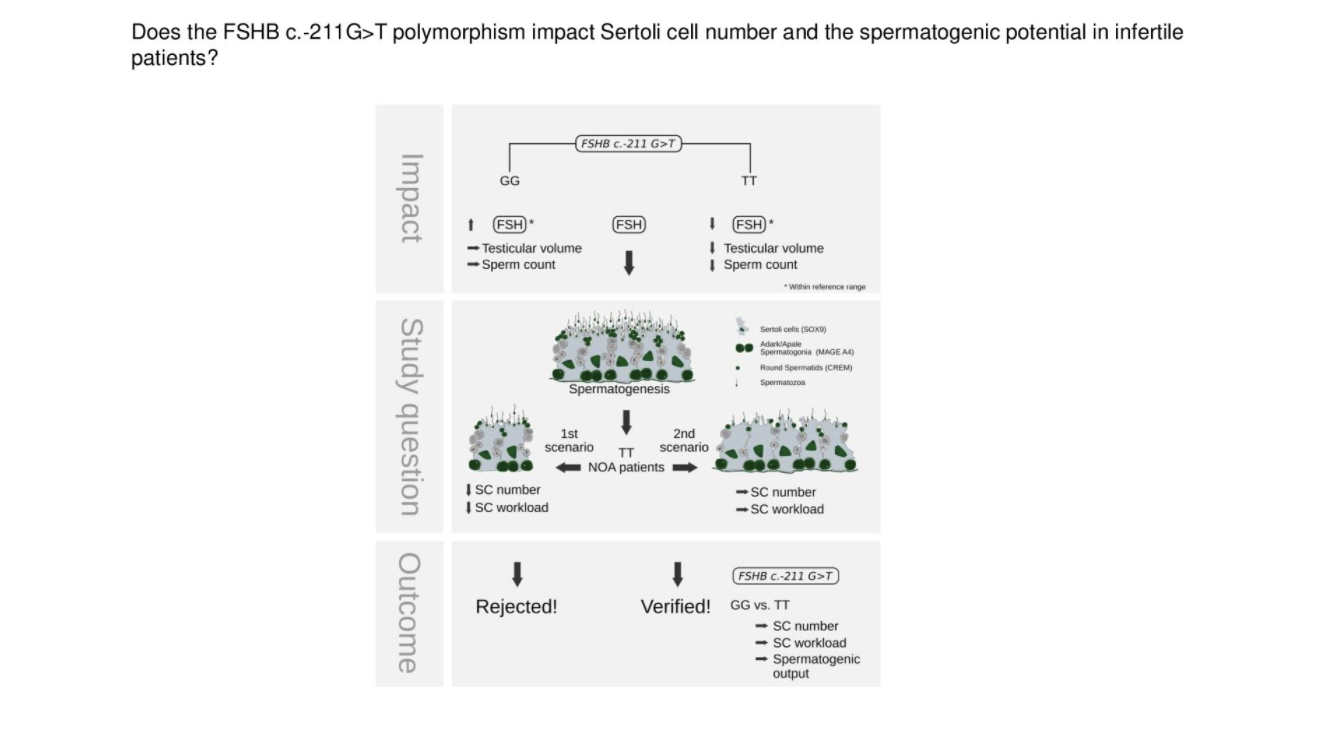
Figure 1. Thesis
Study synopsis. The T‐allele of the FSHB c.‐211G>T polymorphism impacts FSH serum level, testicular volume, and sperm counts significantly. There are two possible scenarios for the impact of the T‐allele on spermatogenesis: 1st scenario: SC number, SCWL and thereby spermatogenic potential is reduced, 2nd scenario: SC number remains constant, SCWL is reduced and thereby spermatogenic potential (SCs are not the limiting factor) is maintained. Study outcome supports scenario 2, for which as a consequence a further stimulation of spermatogenesis in infertile men with a T‐allele by FSH is conceivable
Licensed under: https://creativecommons.org/licenses/by/4.0/
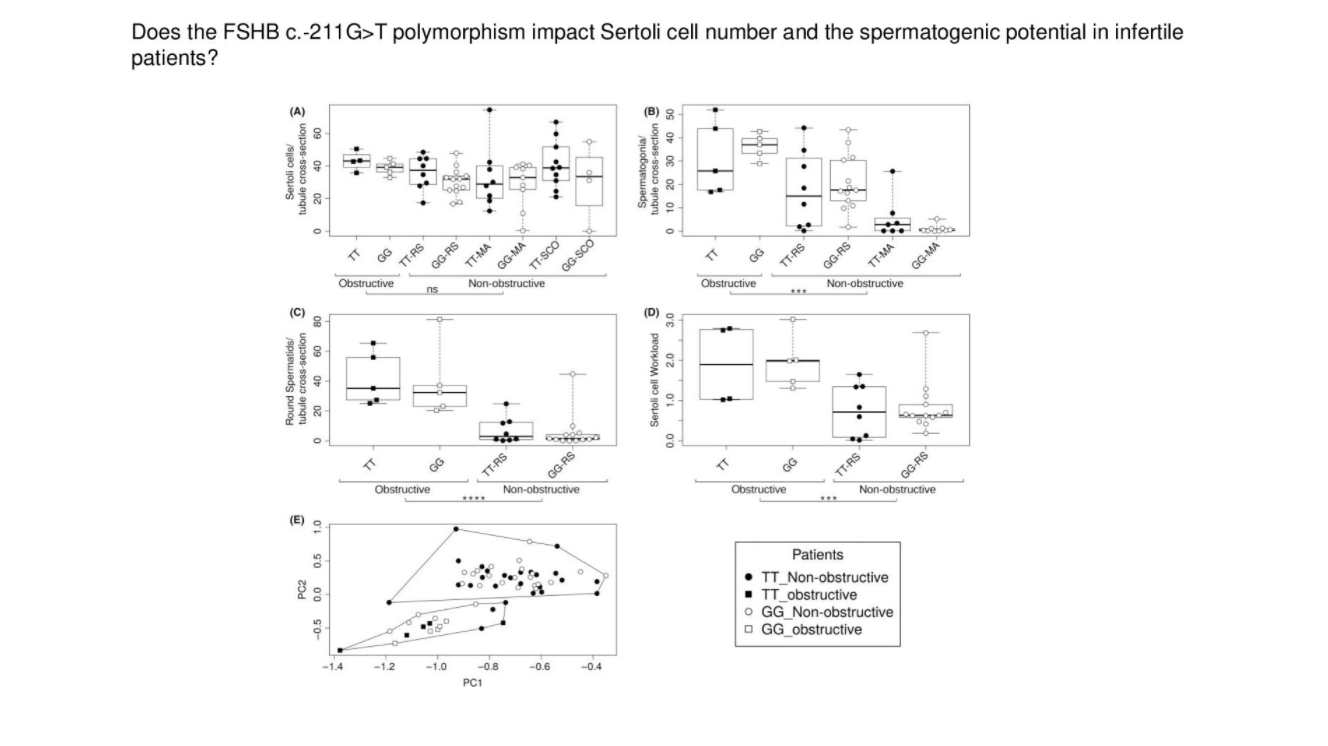
Figure 3. Quantification
Quantification of (A) Sertoli cell number, (B) spermatogonia number, (C) round spermatids number per tubule cross‐section and (D) Sertoli cell workload (SCWL) in patients with NOA and OA stratified by the FSHB c.‐211G>T genotype (E) cluster analysis of all patients, based on min‐max transformed clinical and histological parameters. The cluster analysis proposes an allocation of patients into two clusters (NOA and OA) indicated by the encirclement independent of their corresponding genotype. Whisker dot plots show number of cells per tubule cross‐section, dots present single patient samples, different filling of the icons represent respective genotype in FSHB c.‐211 G > T black: TT; white: GG. Subgroups were formed within the NOA group according to highest achieved grade of spermatogenesis: Round spermatids (RS), meiotic arrest (MA), in case of Sertoli cells only (SCO). Statistical analysis was performed using the Mann‐Whitney U test (P < =.05), ns = P > .05, *** = P ≤ .001, **** = P ≤ .0001
Licensed under: https://creativecommons.org/licenses/by/4.0/
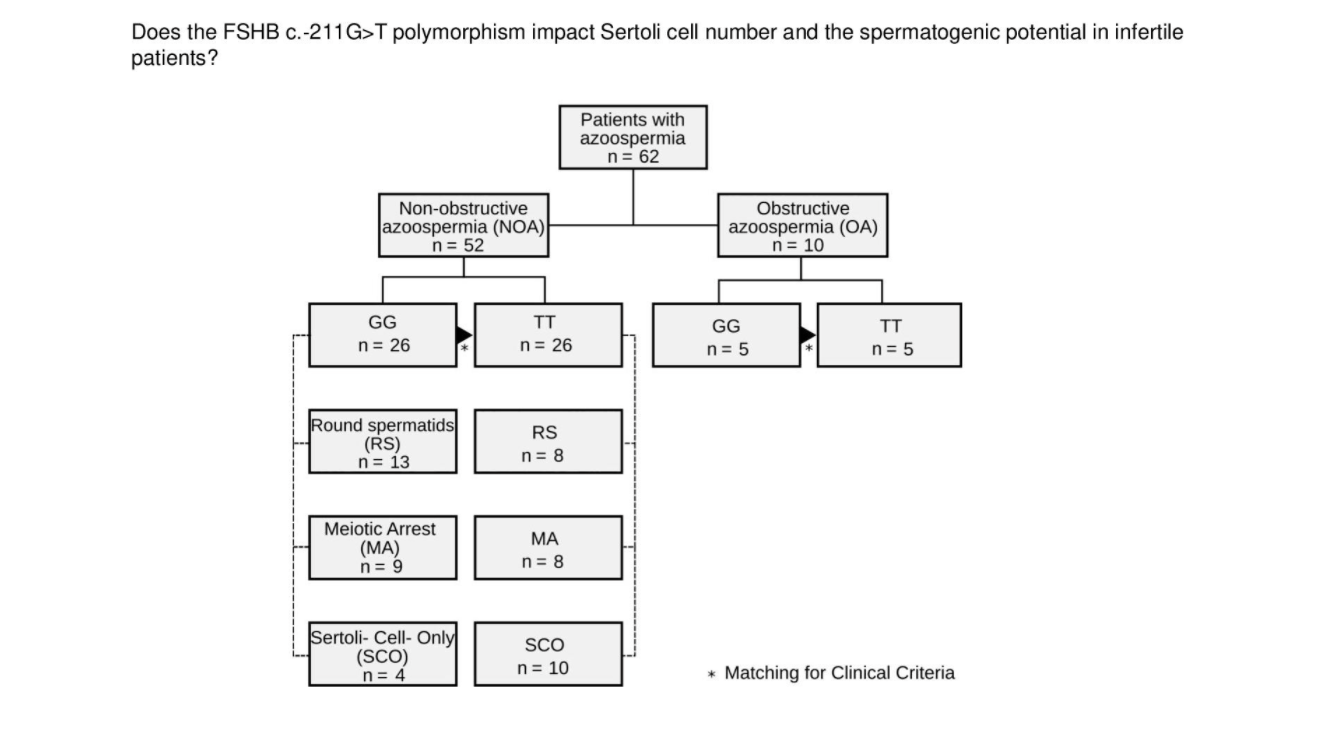
Figure 2. Study Cohort
Study cohort. Selection flowchart for patients with NOA and OA stratified by the FSHB c.‐211G>T genotype and age‐matched
Licensed under: https://creativecommons.org/licenses/by/4.0/
