Molecular Aging Markers in Patients with Klinefelter Syndrome
Eva Pohl, Sina Muschal, Sabine Kliesch, Michael Zitzmann, Julia Rohayem, Jörg Gromoll, Sandra Laurentino, 01.08.2019
Abstract
Molecular aging markers provide the opportunity for biological age determination in humans and to study factors, such as genetic determinants, affecting the ageing process. In males with Klinefelter syndrome (KS, non-mosaic karyotype 47, XXY), which is the most common sex chromosome aneuploidy, age-related morbidity and mortality are increased, and a significantly reduced life span has been observed. The aim of this study was to investigate whether Klinefelter patients exhibit molecular signs of premature ageing. We studied, specifically, age-associated DNA methylation patterns (by pyrosequencing) and relative telomere length (TL; by quantitative polymerase chain reaction) in blood in a cohort of Klinefelter patients (n=178 and 266 for DNA methylation and TL, respectively) aged 18-71 years and compared them to the data of age-matched healthy male (n = 184 and 196 for DNA methylation and TL, respectively) and female controls (n = 50). Age-associated DNA methylation patterns were not indicative of accelerated ageing in Klinefelter men. Significantly longer telomere were found in the young Klinefelter subjects aged 18-24 years (mean=1.51 vs. 1.09 and 1.26 in female and male controls, respectively). However, telomere length in subsequent age groups showed no difference to controls. Gonosomal aneuploidy in Klinefelter syndrome is associated with higher baseline TL at adolescent age, bu comparable TL with progressive age in other age groups.
POHL, Eva, et al. Molecular Aging Markers in Patients with Klinefelter Syndrome. Aging and disease, 2020, 11. Jg., Nr. 3, S. 470.
Publication: http://dx.doi.org/10.14336/AD.2019.0801
 Disclaimer
Disclaimer
The publication Molecular Aging Markers in Patients with Klinefelter Syndrome by Eva Pohl, Sina Muschal, Sabine Kliesch, Michael Zitzmann, Julia Rohayem, Jörg Gromoll, Sandra Laurentino is published under an open access license: https://creativecommons.org/licenses/by/4.0/. Permits unrestricted use,distribution, and reproduction in any medium, provided the original author and source are credited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Pyrosequencing and age prediction
Methylome: Other
Species
| Species |
|---|
| Human |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:control | 184 | Age-matched healthy male participants |
| HP:other: Other | 50 | Healthy female control. |
| HP:klinefelter: Klinefelter Syndrome | 178 | Male-participants were 18-71 years of age |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000081: blood cell | Initially, 32 blood samples served as an independent training set for obtaining an age-prediction formula. Factors for formula: methylation values for cg02228185 (ASPA), cg25809905 (ITGA2B), and cg17861230 (PDE4C). | Human |
Images
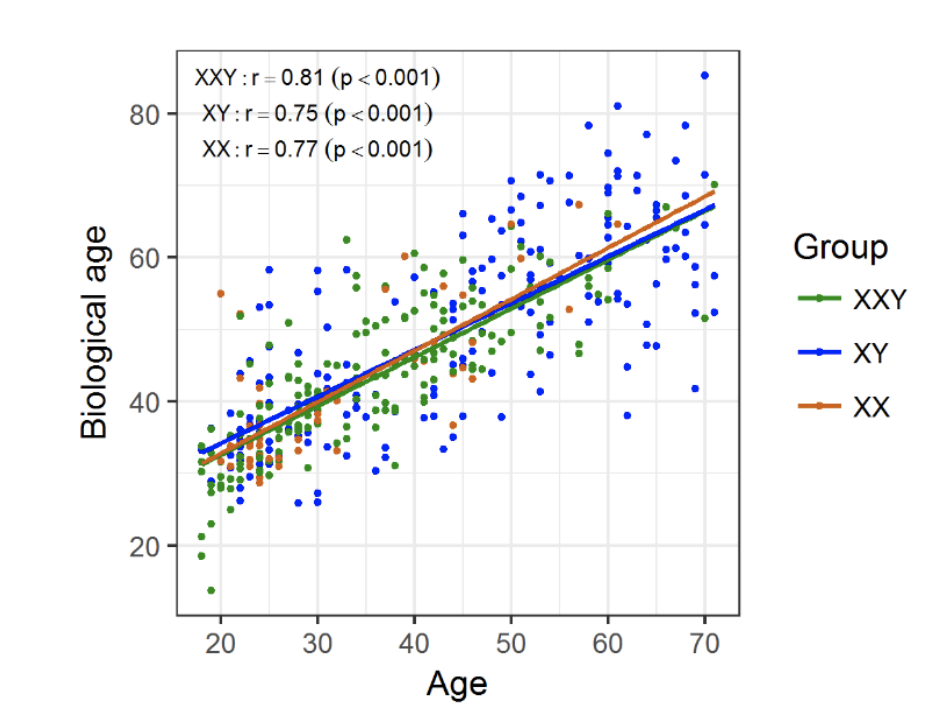
Figure 1. Biological/epigenetic age prediction comparison
Biological/epigenetic age was determined for individuals in the three groups and plotted against chronological age. There was a strong positive linear correlation between the two, regardless of the group.
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 2: Telomere length determination
Genome: Targeted Genotyping
Species
| Species |
|---|
| Human |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:control | 196 | Age-matched healthy male participants |
| HP:other: Other | 50 | Female controls |
| HP:klinefelter: Klinefelter Syndrome | 266 | Male-participants were 18-71 years of age |
Images
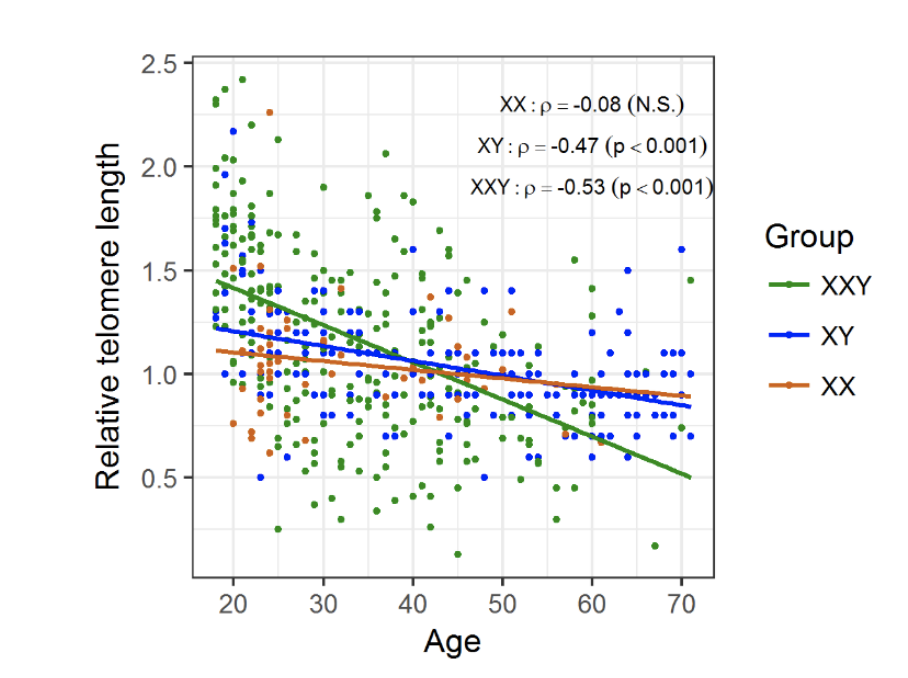
Figure 2. Changes in telomere length with age, according to group
Telomeres suffer attrition with age, regardless of genotype, however telomere shortening shows a steeper decrease in KS men (m = -0.02) compared to XY males (m = - 0.007; p < 0.001) and XX females (m = -0.004; p < 0.01) probands.
Licensed under: https://creativecommons.org/licenses/by/4.0/
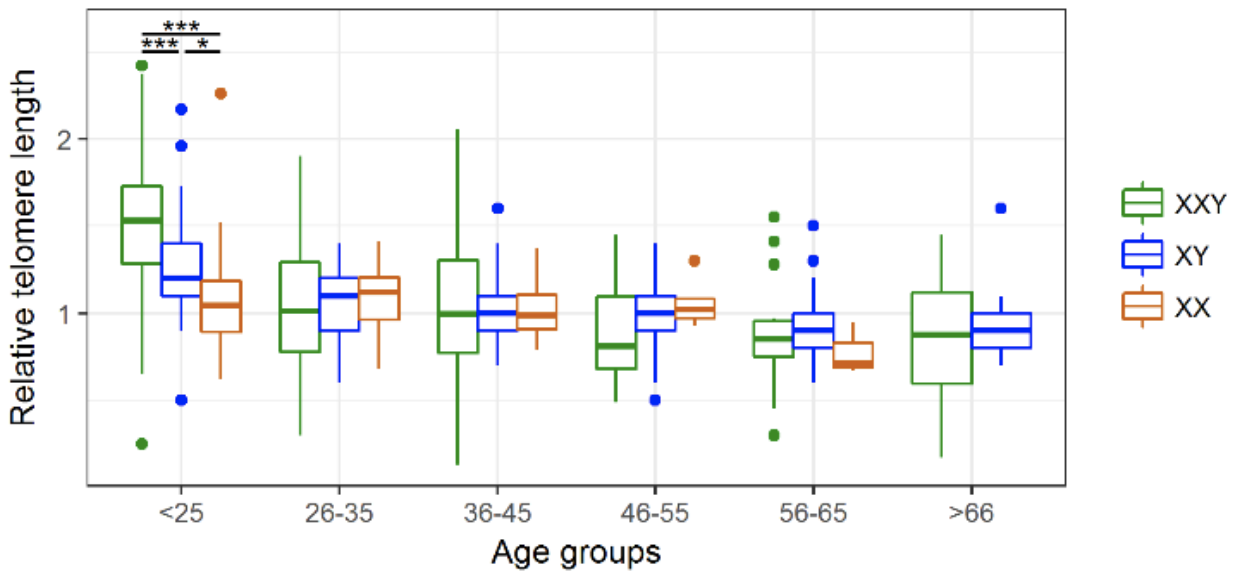
Figure 3. Age-group associated changes in telomere length.
Comparison of the average relative Telomere length (rTL) in the three study groups reveals that KS patients present significantly longer telomeres than both XY males and XX females only in the youngest age group. * p < 0.05, *** p < 0.001.
Licensed under: https://creativecommons.org/licenses/by/4.0/
