Testicular blood supply is altered in the 41,XXY* Klinefelter syndrome mouse model
Joachim Wistuba, Cristin Beumer, Ann-Sophie Warmeling, Reinhild Sandhowe-Klaverkamp, Jörg Stypmann, Michael Kuhlmann, Richard Holtmeier, Oliver S. Damm, Frank Tüttelmann, Jörg Gromoll, 01.09.2020
Abstract
Hypergonadotropic hypogonadism is a major feature of Klinefelter syndrome (KS), assumed to be caused by testicular hormone resistance. It was previously shown that intratesticular testosterone levels in vivo and Leydig cell function in vitro seem to be normal indicating other functional constraints. We hypothesized that impaired testicular vascularization/blood flow could be a co-factor to the observed hypergonadotropic hypogonadism. We evaluated the testicular vascular system by measuring blood vessel sizes during postnatal development and testis blood flow in adult 41,XXY* mice. Proportional distribution and size of blood vessels were analyzed during testicular development (1, 3, 5, 7, 10, 21 dpp, 15 wpp). While ratios of the vessel/testis area were different at 15 wpp only, a lower number of smaller and mid-sized blood vessels were detected in adult KS mice. For testicular blood flow determination we applied contrast enhanced ultrasound. Floating and reperfusion time for testicular blood flow was increased in 41,XXY* mice (floating: XY* 28.8 ± 1.69 s vs XXY* 44.6 ± 5.6 s, p = 0.0192; reperfusion XY* 19.7 ± 2.8 s vs XXY*: 29.9 ± 6.2 s, p = 0.0134), indicating a diminished blood supply. Our data strengthen the concept that an impaired vascularization either in conjunction or as a result of altered KS testicular architecture contributes to hormone resistance.
WISTUBA, Joachim, et al. Testicular blood supply is altered in the 41, XX Y* Klinefelter syndrome mouse model. Scientific Reports, 2020, 10. Jg., Nr. 1, S. 1-10.
Publication: https://doi.org/10.1038/s41598-020-71377-0
 Disclaimer
Disclaimer
The publication Testicular blood supply is altered in the 41,XXY* Klinefelter syndrome mouse model by Joachim Wistuba, Cristin Beumer, Ann-Sophie Warmeling, Reinhild Sandhowe-Klaverkamp, Jörg Stypmann, Michael Kuhlmann, Richard Holtmeier, Oliver S. Damm, Frank Tüttelmann, Jörg Gromoll is published under an open access license: http://creativecommons.org/licenses/by/4.0/. Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Testicular vascularization during development
Other: Immunohistochemistry
Species
| Species |
|---|
| Mouse |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:control | 52 | Histological evaluation at different developmental stages: 1 dpp (XY* n = 7), 3 dpp (XY* n = 5), 5 dpp (XY* n = 6), 7 dpp (XY* n = 6), 10 dpp (XY* n = 5), 14 dpp (XY* n = 5), 21 dpp (XY* n = 6) dpp and 15–16 (XY* n = 12) wpp. |
| HP:klinefelter: Klinefelter Syndrome | 45 | Histological evaluation at different developmental stages: at 1 dpp (XXY*: n = 5), 3 dpp (XXY*: n = 5), 5 dpp (XXY*: n = 5), 7 dpp (XXY*: n = 5), 10 dpp (XXY*: n = 5,), 14 dpp (XXY*: n = 5,), 21 dpp (XXY*: n = 5) dpp and 15–16 (XXY*: n = 10) wpp |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | Extracted at different developmental stages | Mouse |
Images
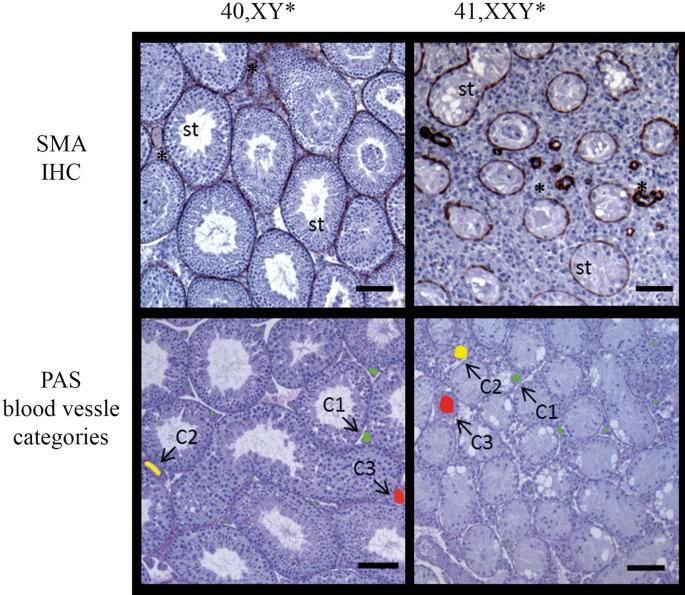
Figure 1. Vessel categories for vascularization analyses in 40 XY* and 41,XXY* mice.
Upper panel: testicular cross-sections stained for alpha-smooth muscle actin (SMA) to visualize blood vessels (stars) between the seminiferous tubules (st). Such sections were used for quantitative analyses. Lower panel: PAS-stained testicular cross-sections with vessels colored for illustration of size categories (< 80 µm2: green, 80–1,000 µm2: yellow, > 1,000 µm2g: red). Scale bar equals 200 µm.
Licensed under: http://creativecommons.org/licenses/by/4.0/
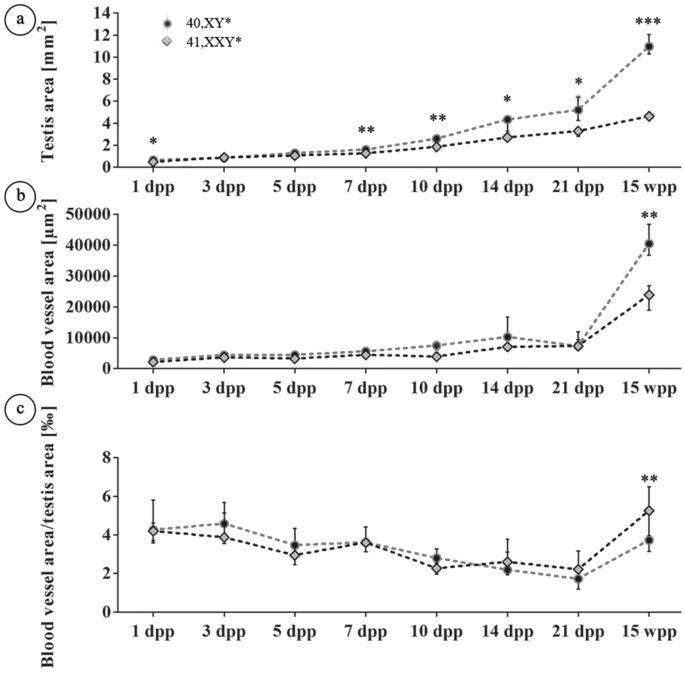
Figure 2. Analysis of testicular vascularization
Analysis of testicular vascularization over the postnatal development (1, 3, 5, 7, 10, 14, 21 dpp, 15 wpp) in 40,XY* and 41,XXY* mice. Testicular cross sections stained immunohstochemically for SMA were analyzed for the total area covered by blood vessels (blood vessel area) and the whole cross sectional area (testis area) as described by Tüttelmann et al.18. (a) Testis area. (b) Blood vessel area. (c) Ratios of vessel area/testis area. In (a–c), medians with interquartile ranges are shown; *p < 0.05; **p < 0.01; ***p < 0.001.
Licensed under: http://creativecommons.org/licenses/by/4.0/

Figure 3. Vessel size distribution in testes of 40,XY* and 41,XXY* mice.
Blood vessels were grouped according to the three categories < 80 µm2, 80–1,000 µm2 and > 1,000 µm2. The blood vessel area/testis area ratio was determined considering always only vessels of the respective size categories. (a) Area covered by vessels assigned to the three size categories in 15 wpp testes. (b) Blood vessel area/testis area ratio considering vessels of three size categories in 15 wpp testes. All data are presented as median values with interquartile ranges. Dot plots additionally include the individual values obtained from each testis analyzed; *p < 0.05; **p < 0.01; ***p < 0.001.
Licensed under: http://creativecommons.org/licenses/by/4.0/
Data set 2: Determination of testicular blood flow by contrast enhanced ultrasound analysis (CEUS)
Other: Functional Study
Species
| Species |
|---|
| Mouse |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:control | 12 | Mice (XY* n = 12) aged 15–16 weeks were analyzed. |
| HP:klinefelter: Klinefelter Syndrome | 10 | Mice (XXY*: n = 10) aged 15–16 weeks were analyzed. |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001102: blood vessel | Adult | Considering the measure blood flow in testis in vivo. | Mouse | |
| BTO_0001363: testis | Mouse |
Images
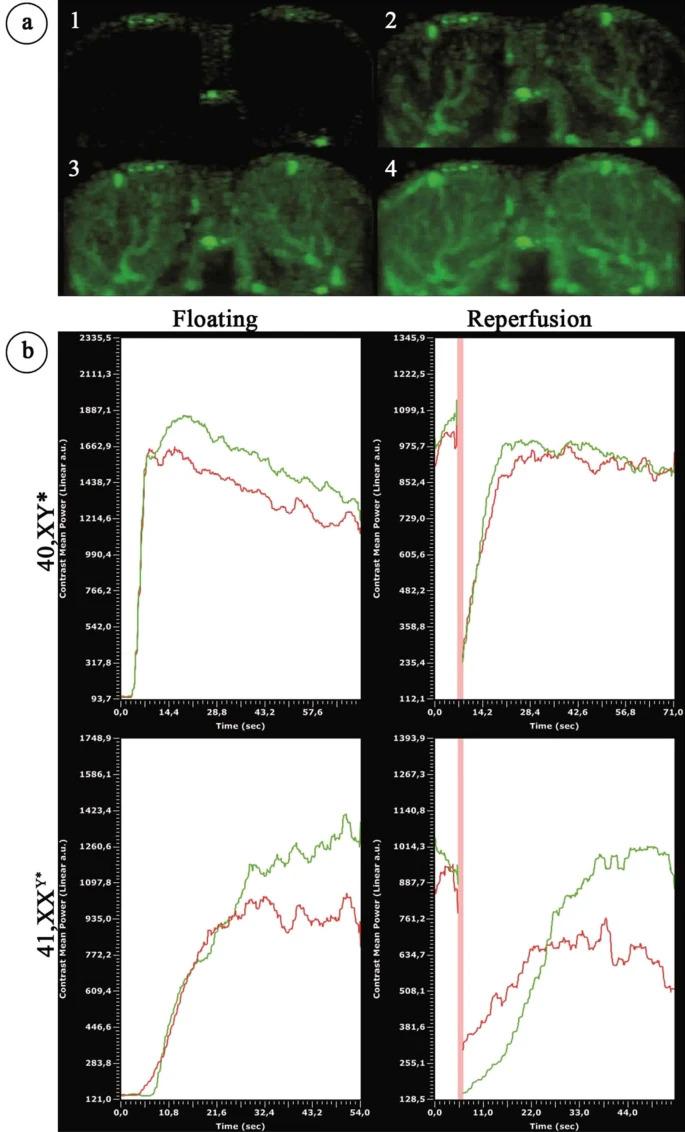
Figure 4. Contrast-enhanced ultrasound analysis of testicular blood flow in adult 40,XY* and 41,XXY* mice.
(a) Perfusion of the testes by contrast agent: after injection before the contrast agent reached the testis (1). Contrast agent appears in the testicular area (2) and the covered area increases (3) until the state of maximal filling after perfusion: “floating” is reached (4). (b) Left upper and lower graph: graphical illustration of blood flow by ultrasound signal intensity in 40,XY* and 41,XXY* mice. Right upper and lower graph: after floating, the contrast agent signal was destroyed by a boost (high power ultrasound pulse) before the testes was flooded again with contrast agent (“reperfusion”). Floating as well as reperfusion duration (“time to peak”) served as the functional readout for the testicular blood flow. Green = signal measured in right; red in left testis. The red vertical line reflects the boost.
Licensed under: http://creativecommons.org/licenses/by/4.0/
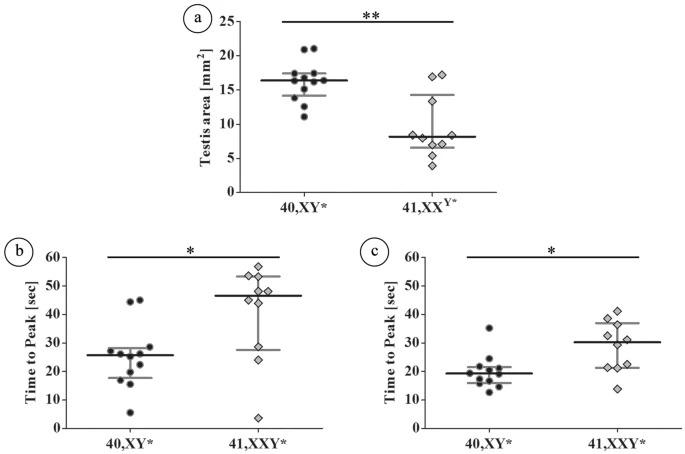
Figure 5. Quantitative analysis of the testicular blood flow in adult (15–16 wpp) 40,XY* and 41,XXY* mice by contrast-enhanced ultrasound.
Testicular perfusion by contrast enhanced ultrasound: (a) testicular area reflecting the different testis sizes between both groups; (b) time to peak of floating was significantly lower in wild type males; (c) time to peak of reperfusion was also significantly lower in wild type males. In both measurements the increased time for testicular perfusion in XXY* mice indicates hampered blood flow. Black dots: 40,XY*, grey diamonds: 41,XXY*. *p < 0.05; **p < 0.01.
Licensed under: http://creativecommons.org/licenses/by/4.0/
