Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca2+ signaling
Christian Schiffer, Steffen Rieger, Christoph Brenker, Samuel Young, Hussein Hamzeh, Dagmar Wachten, Frank Tüttelmann, Albrecht Röpke, U. Benjamin Kaupp, Tao Wang, Alice Wagner, Claudia Krallmann, Sabine Kliesch, Carsten Fallnich and Timo Strünker, 19.01.2020
Abstract
Navigation of sperm in fluid flow, called rheotaxis, provides long‐range guidance in the mammalian oviduct. The rotation of sperm around their longitudinal axis (rolling) promotes rheotaxis. Whether sperm rolling and rheotaxis require calcium (Ca2+) influx via the sperm‐specific Ca2+ channel CatSper, or rather represent passive biomechanical and hydrodynamic processes, has remained controversial. Here, we study the swimming behavior of sperm from healthy donors and from infertile patients that lack functional CatSper channels, using dark‐field microscopy, optical tweezers, and microfluidics. We demonstrate that rolling and rheotaxis persist in CatSper‐deficient human sperm. Furthermore, human sperm undergo rolling and rheotaxis even when Ca2+ influx is prevented. Finally, we show that rolling and rheotaxis also persist in mouse sperm deficient in both CatSper and flagellar Ca2+‐signaling domains. Our results strongly support the concept that passive biomechanical and hydrodynamic processes enable sperm rolling and rheotaxis, rather than calcium signaling mediated by CatSper or other mechanisms controlling transmembrane Ca2+ flux.
SCHIFFER, Christian, et al. Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca2+ signaling. The EMBO journal, 2020, 39. Jg., Nr. 4, S. e102363.
Publication: https://doi.org/10.15252/embj.2019102363
 Disclaimer
Disclaimer
The publication Rotational motion and rheotaxis of human sperm do not require functional CatSper channels and transmembrane Ca2+ signaling by Christian Schiffer, Steffen Rieger, Christoph Brenker, Samuel Young, Hussein Hamzeh, Dagmar Wachten, Frank Tüttelmann, Albrecht Röpke, U. Benjamin Kaupp, Tao Wang, Alice Wagner, Claudia Krallmann, Sabine Kliesch, Carsten Fallnich and Timo Strünker is published under an open access no derivatives license: : https://creativecommons.org/licenses/. Permits non-commercial use, distribution and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: The expression of pore‐forming CatSper subunits is not strictly interdependent
Proteome: Immunohistochemistry
Species
| Species |
|---|
| Human |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:0000789: Infertility | 5.0 | Patients suffering from a homozygous deletion of contiguous genes on chromosome 15, including the CATSPER2 gene. |
| HP:control |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm |
Images
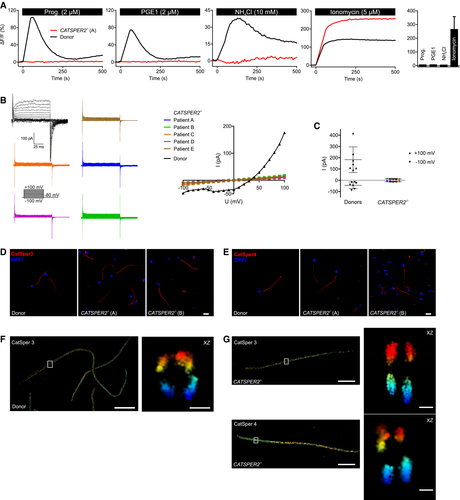
Figure 1. Characterization of CATSPER2‐deficient human sperm
A.</br>Representative Ca2+ signals in sperm from a patient with deafness‐infertility syndrome lacking functional CatSper channels (CATSPER2−/−; red) and a healthy donor (black), evoked by progesterone, PGE1, NH4Cl, or ionomycin. NH4Cl increases the intracellular pH. Bar graph: Amplitudes (n = 4; mean ± SD) of Ca2+ signals in CATSPER2−/− sperm.</br>B.</br>Representative monovalent CatSper currents in CATSPER2−/− sperm (blue, green, orange, purple, brown) and in sperm from a healthy donor (black), and corresponding current‐voltage relationship (right). The membrane voltage was stepped from −100 to +100 mV in increments of 10 mV from a holding potential of −80 mV.</br>C.</br>Outward and inward current amplitudes (mean ± SD) at + 100 mV and ‐100 mV, respectively, in CATSPER2−/− sperm (color code: panel B) and sperm from healthy donors (black).</br>D, E.</br>Representative immunocytochemical staining of control sperm from healthy donors and CATSPER2−/− sperm from DIS patients using antibodies directed against CatSper 3 (D) or CatSper 4 (E); DNA was labeled with DAPI (blue). Scale bars represent 10 μm.</br>F.</br>3D‐STORM image in xy projection of sperm from a healthy donor labeled with the anti‐CatSper 3 antibody (left). Axial projection of the boxed region (right). Scale bars represent 5 μm in xy projections and 200 nm in axial projections.</br>G.</br>3D‐STORM images in xy projection of CATSPER2−/− sperm (left) labeled with the anti‐CatSper 3 (upper panel) or anti‐CatSper 4 (lower panel) antibody. Axial projection of the boxed regions (right). Scale bars represent 5 μm in xy projections and 200 nm in axial projections.
Licensed under: https://creativecommons.org/licenses/
Data set 2: Human sperm do not require functional CatSper channelsfor longitudinal rolling
Proteome: Functional Study
Species
| Species |
|---|
| Human |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm |
Images
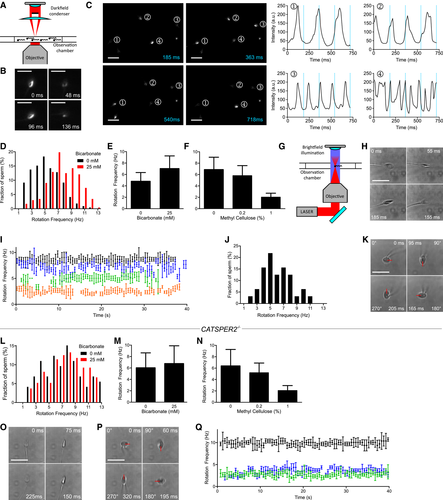
Figure 2.Analysis of longitudinal rolling of human sperm
A. </br>Experimental setup for population analysis by dark‐field microscopy.</br>B.</br>Dark‐field microscopy of a single sperm cell; shown are single frames obtained at t = 0, 48, 96, and 136 ms. Scale bar = 25 μm.</br>C.</br>Dark‐field imaging of a sperm population; left: single frames at t = 185, 363, 540, and 718 ms. Sperm selected for analysis are highlighted (1–4). Right: temporal change in the brightness (blinking) of sperm heads. The blue lines correspond to the time‐points of the single frames. Scale bar = 25 μm.</br>D.</br>Representative distribution of rotation frequencies of freely swimming sperm incubated under non‐capacitating (0 mM bicarbonate; black; n = 218) and capacitating (25 mM bicarbonate; red; n = 232) conditions determined by dark‐field imaging.</br>E.</br>Rolling frequency (mean ± SD) of sperm incubated under non‐capacitating (0 mM bicarbonate, n = 1,455; three experiments) and capacitating (25 mM bicarbonate, n = 1,097, eight experiments) conditions.</br>F.</br>Rolling frequency (mean ± SD) of freely swimming sperm in 0 (n = 1,175; five experiments), 0.2 (n = 832; three experiments), and 1% (n = 599; three experiments) methyl cellulose (w/v).</br>G.</br>Experimental setup for the laser‐based optical tweezer.</br>H.</br>Bright‐field images of an optically trapped sperm cell obtained at t = 0, 55, 155, and 185 ms. Scale bar represents 10 μm.</br>I.</br>Representative time‐course of the rotation frequency of trapped sperm (each sperm cell is represented by a different color). Error bars indicate the full width at half prominence of the frequency peaks determined by the fast Fourier analysis.</br>J.</br>Distribution of rotation frequencies in trapped sperm (n = 32; mean frequency ± SD 6.0 ± 2.1 Hz).</br>K.</br>Image series of a sperm cell trapped parallel to the optical axis; images were obtained at t = 0, 95, 165, and 205 ms. The red bar indicates the 360° rotation of the tip of the head. Scale bar represents 10 μm.</br>L.</br>Representative distribution of rotation frequencies of freely swimming CatSper‐deficient sperm incubated under non‐capacitating (0 mM bicarbonate; black; n = 73) and capacitating conditions (25 mM bicarbonate; red; n = 272).</br>M.</br>Rolling frequency (mean ± SD) of freely swimming CATSPER2−/− sperm incubated under non‐capacitating (0 mM bicarbonate, n = 1,009; four experiments) and capacitating (25 mM bicarbonate, n = 946; seven experiments) conditions.</br>N.</br>Rolling frequency (mean ± SD) of freely swimming CATSPER2−/− sperm in 0 (n = 457; four experiments), 0.2 (n = 389; two experiments), and 1% (n = 187; two experiments) methyl cellulose (w/v).</br>O.</br>Image series of a CATSPER2−/− sperm cell optically trapped perpendicular to the optical axis; images were obtained at t = 0, 75, 150, and 225 ms. Scale bar represents 10 μm.</br>P.</br>Image series of a CATSPER2−/− sperm cell optically trapped parallel to the optical axis; images were obtained at t = 0, 60, 195, and 320 ms. The red bar indicates the 360° rotation of the tip of the head. Scale bar represents 10 μm.</br>Q.</br>Representative time courses of the rotation frequencies of optically trapped CATSPER2−/− sperm (each sperm is represented in a different color). Error bars indicate the full width at half prominence of the frequency peaks determined by the fast Fourier analysis.
Licensed under: https://creativecommons.org/licenses/
Data set 3: Longitudinal rolling of human sperm does not require an influx of Ca2+
Proteome: Functional Study
Species
| Species |
|---|
| Human |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm |
Images
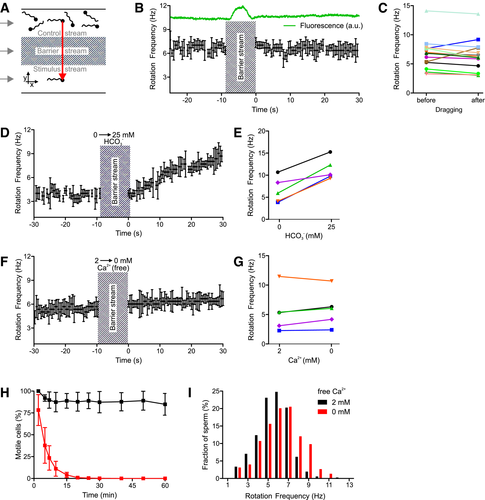
Figure 3.The action of bicarbonate and Ca2+ on longitudinal rolling of human sperm
A.</br>Experimental setup to subject optically trapped sperm to different conditions in a three‐channel microfluidic capillary.</br>B.</br>Rotation frequency of a trapped sperm cell before and after dragging across the barrier stream. The green trace indicates the fluorescence of fluorescein included in the barrier stream. Error bars indicate the full width at half prominence of the frequency peaks determined by the fast Fourier analysis.</br>C.</br>Paired plot of rotation frequencies of individual sperm cells before and after dragging across the barrier stream.</br>D.</br>Rotation frequency of a trapped sperm cell before and after dragging from the control stream containing 0 mM bicarbonate into the stimulus stream containing 25 mM bicarbonate. Error bars indicate the full width at half prominence of the frequency peaks determined by the fast Fourier analysis.</br>E.</br>Paired plot of rotation frequencies of individual sperm at 0 and 25 mM bicarbonate.</br>F.</br>Rotation frequency of a trapped sperm cell in the presence and absence of extracellular Ca2+. Error bars indicate the full width at half prominence of the frequency peaks determined by the fast Fourier analysis.</br>G.</br>Paired plot of rotation frequencies in the presence and absence of extracellular Ca2+.</br>H.</br>Fraction of motile sperm (mean ± SD) in a sperm population incubated in the presence (black) and absence (at t = 0) of extracellular Ca2+ (red; n ≥ 5).</br>I.</br>Distribution of rotation frequencies in populations of freely swimming sperm in the presence (black, n = 335) and absence (red, n = 224) of extracellular Ca2+
Licensed under: https://creativecommons.org/licenses/
Data set 4: CatSper‐deficient human sperm display rheotaxis
Proteome: Functional Study
Species
| Species |
|---|
| Human |
| Mouse |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:0012206: Abnormal sperm motility | CatSper‐deficient sperm. | |
| HP:control |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm | Human | ||||
| CL_0000019: sperm | Mouse |
Images
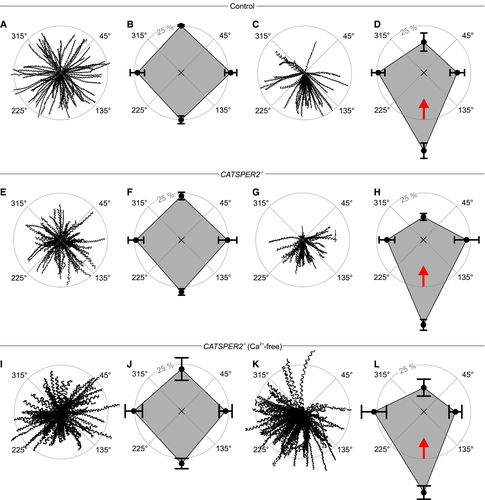
Figure 4.Rheotaxis of human sperm
A.</br>Trajectories of human sperm in the absence of a fluid flow. Sperm were tracked for 1.86 s. The starting point of each trajectory was centered to the origin of a coordinate system, represented by the intersection of the dotted lines in the center of the circle.</br>B.</br>Spider‐web plot of the mean (± SD) relative frequencies of sperm swimming with an angular direction of 315°–45°, 45°–135°, 135°–225°, and 225°–315° (n = 7; 1,301 sperm) in the absence of a fluid flow.</br>C.</br>Representative trajectories of human sperm in the presence of a fluid flow.</br>D.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions (n = 7; 1,083 sperm) in the presence of a fluid flow. The red arrow indicates the flow direction.</br>E.</br>Representative trajectories of CATSPER2−/− sperm in the absence of a fluid flow.</br>F.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions of CATSPER2−/− sperm in the absence of a fluid flow (n = 6; 1,068 sperm).</br>G.</br>Trajectories of CATSPER2−/− sperm in the presence of a fluid flow.</br>H.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions of CATSPER2−/− sperm in the presence of a fluid flow (n = 7; 1,068 sperm). The red arrow indicates the fluid flow direction.</br>I.</br>Representative trajectories of CATSPER2−/− sperm in Ca2+‐free buffer in the absence of a fluid flow.</br>J.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions (n = 4; 442 sperm) of CATSPER2−/− sperm in Ca2+‐free buffer in the absence of a fluid flow.</br>K.</br>Representative trajectories of CATSPER2−/− sperm in Ca2+‐free buffer in the presence of a fluid flow.</br>L.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions (n = 4; 620 sperm) of CATSPER2−/− sperm in Ca2+‐free buffer in the presence of a fluid flow. The red arrow indicates the flow direction.
Licensed under: https://creativecommons.org/licenses/
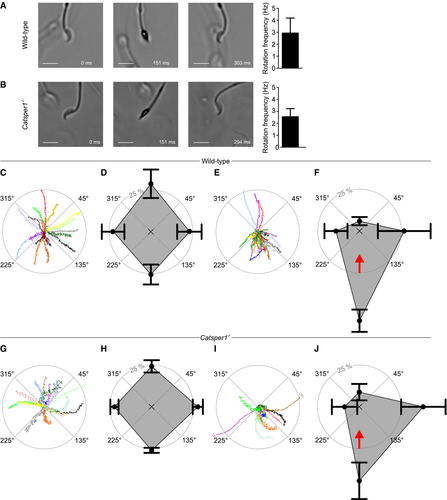
Figure 5.Rolling behavior and rheotaxis of mouse sperm
A.</br>Left: Bright‐field image series of a freely swimming wild‐type sperm cell at t = 0, 151, and 303 ms. Scale bar represents 10 μm. Right: rotation frequency (mean ± SD) of freely swimming wild‐type sperm (n = 24, three experiments).</br>B.</br>Left: Bright‐field image series of a freely swimming Catsper1−/− sperm cell at t = 0, 151, and 294 ms. Scale bar represents 10 μm. Right: rotation frequency (mean ± SD) of freely swimming Catsper1−/− sperm (n = 24, three experiments).</br>C.</br>Representative trajectories of wild‐type sperm in the absence of a fluid flow. The starting point of each trajectory was centered to the origin of a coordinate system, represented by the intersection of the dotted lines in the center of the circle. Each color represents one trajectory.</br>D.</br>Spider‐web plot of the mean (± SD) relative frequencies of sperm swimming with an angular direction of (binning: 315°–45°, 45°–135°, 135°–225°, and 225°–315° (n = 3; 127 sperm) in the absence of a fluid flow.</br>E.</br>Representative trajectories of wild‐type sperm in the presence of a fluid flow.</br>F.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions (n = 3; 175 sperm) of wild‐type sperm in the presence of a fluid flow. The red arrow indicates the flow direction.</br>G.</br>Representative trajectories of Catsper1−/− sperm in the absence of a fluid flow. The starting point of each trajectory was centered to the origin of a coordinate system, represented by the intersection of the dotted lines in the center of the circle. Trajectories are magnified by a factor of 2.05 with respect to the plots C and E to compensate for the reduced swimming speed of the Catsper1−/− sperm.</br>H.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions of Catsper1−/− sperm (n = 4; 297 sperm) in the absence of a fluid flow.</br>I.</br>Representative trajectories of Catsper1−/− sperm in the presence of a fluid flow; trajectories are magnified by a factor of 2.05 with respect to the plots C and E to compensate for the reduced swimming speed of the Catsper1−/− sperm, and two trajectories were truncated (indicated by two parallel lines).</br>J.</br>Spider‐web plot of the mean (± SD) relative frequencies of angular swimming directions (n = 4; 261 sperm) of Catsper1−/− sperm in the presence of a fluid flow. The red arrow indicates the flow direction.
Licensed under: https://creativecommons.org/licenses/
