A de novo paradigm for male infertility
Oud MS, Smits RM, Smith HE, Mastrorosa FK, Holt GS, Houston BJ, de Vries PF, Alobaidi BKS, Batty LE, Ismail H, Greenwood J, Sheth H, Mikulasova A, Astuti GDN, Gilissen C, McEleny K, Turner H, Coxhead J, Cockell S, Braat DDM, Fleischer K, D'Hauwers KWM, Schaafsma E, 10.01.2022
Abstract
De novo mutations are known to play a prominent role in sporadic disorders with reduced fitness. We hypothesize that de novo mutations play an important role in severe male infertility and explain a portion of the genetic causes of this understudied disorder. To test this hypothesis, we utilize trio-based exome sequencing in a cohort of 185 infertile males and their unaffected parents. Following a systematic analysis, 29 of 145 rare (MAF < 0.1%) protein-altering de novo mutations are classified as possibly causative of the male infertility phenotype. We observed a significant enrichment of loss-of-function de novo mutations in loss-of-function-intolerant genes (p-value = 1.00 × 10-5) in infertile men compared to controls. Additionally, we detected a significant increase in predicted pathogenic de novo missense mutations affecting missense-intolerant genes (p-value = 5.01 × 10-4) in contrast to predicted benign de novo mutations. One gene we identify, RBM5, is an essential regulator of male germ cell pre-mRNA splicing and has been previously implicated in male infertility in mice. In a follow-up study, 6 rare pathogenic missense mutations affecting this gene are observed in a cohort of 2,506 infertile patients, whilst we find no such mutations in a cohort of 5,784 fertile men (p-value = 0.03). Our results provide evidence for the role of de novo mutations in severe male infertility and point to new candidate genes affecting fertility.
Oud MS, Smits RM, Smith HE, Mastrorosa FK, Holt GS, Houston BJ, de Vries PF, Alobaidi BKS, Batty LE, Ismail H, Greenwood J, Sheth H, Mikulasova A, Astuti GDN, Gilissen C, McEleny K, Turner H, Coxhead J, Cockell S, Braat DDM, Fleischer K, D'Hauwers KWM, Schaafsma E; Genetics of Male Infertility Initiative (GEMINI) consortium; Nagirnaja L, Conrad DF, Friedrich C, Kliesch S, Aston KI, Riera-Escamilla A, Krausz C, Gonzaga-Jauregui C, Santibanez-Koref M, Elliott DJ, Vissers LELM, Tüttelmann F, O'Bryan MK, Ramos L, Xavier MJ, van der Heijden GW, Veltman JA. A de novo paradigm for male infertility. Nat Commun. 2022 Jan 10;13(1):154. doi: 10.1038/s41467-021-27132-8. PMID: 35013161; PMCID: PMC8748898.
Publication: https://doi.org/10.1038/s41467-021-27132-8 Repository: https://ega-archive.org/studies/EGAS00001005417
 Disclaimer
Disclaimer
The publication A de novo paradigm for male infertility by Oud MS, Smits RM, Smith HE, Mastrorosa FK, Holt GS, Houston BJ, de Vries PF, Alobaidi BKS, Batty LE, Ismail H, Greenwood J, Sheth H, Mikulasova A, Astuti GDN, Gilissen C, McEleny K, Turner H, Coxhead J, Cockell S, Braat DDM, Fleischer K, D'Hauwers KWM, Schaafsma E is published under an open access license: https://creativecommons.org/licenses/by/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Discovery of de novo mutations in infertile male trios
Exome: Whole Exome Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001202: saliva | A slightly alkaline secretion of water, mucin, protein, salts, and often a starch-splitting enzyme, as ptyalin, that is secreted into the mouth by salivary glands, lubricates ingested food, and often begins the breakdown of starches. | Human | ||
| BTO_0000089: blood | The fluid that circulates in the heart, arteries, capillaries, and veins of a vertebrate animal carrying nourishment and oxygen to and bringing away waste products from all parts of the body. | Human |
Images
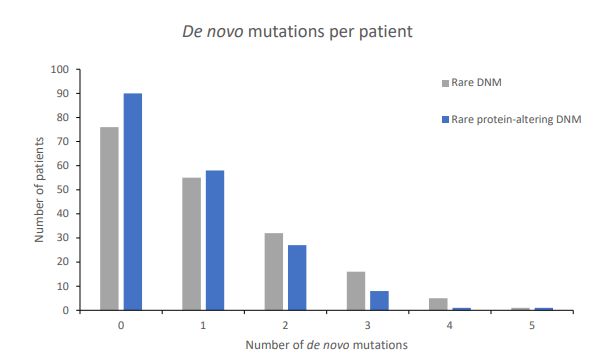
Figure 1: Distribution of 192 de novo mutations in 185 patients.
Distribution of rare de novo mutations (DNM) in all patients (grey) and distribution of rare protein-altering DNMs in all patients (blue).
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 2: Intolerance analysis of genes with de novo loss-of-function mutations
Exome: Whole Exome Sequencing
Images
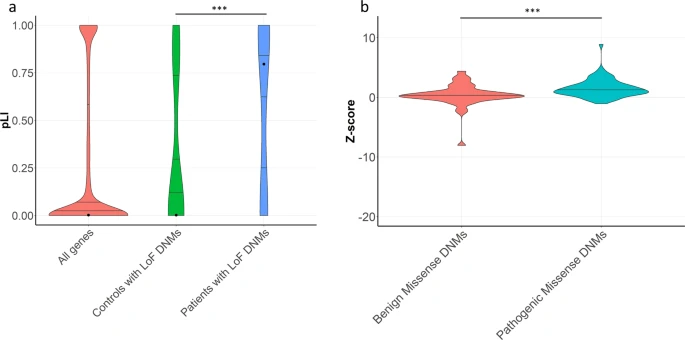
Figure 2: Analysis of the intolerance to loss-of-function and missense variation in genes with de novo mutations.
Violin plot with quantile lines showing pLI scores in all genes in gnomAD (red), all genes affected by rare protein-altering loss-of-function (LoF) de novo mutations (DNMs) in a control population (http://de novo-db.gs.washington.edu/de novo-db/) (green) and in all genes with a rare protein-altering LoF DNM in our trio cohort (blue). Using the permutation-based, nonparametric test defined by Lelieveld et al. 64 a significant enrichment of LoF DNMs in LoF-intolerant genes in patient cohort was detected in comparison to the number of LoF in fertile control cohort (DNM LoF mutations in patients n = 17, median pLI in patients with male infertility = 0.80, DNM LoF mutations in controls n = 21, median pLI in controls = 3.75 × 10−5, p value = 1.00 × 10−5, N simulations = 100,000). The black dot indicates median pLI scores. b Violin plot with quantile lines showing the distribution of Z-scores for genes with predicted benign (n = 59) and pathogenic missense DNMs (n = 63) in infertile patients. A significant increase in predicated pathogenic DNMs in missense-intolerant genes was detected compared to benign missense DNM (Two-sided Mann–Whitney U test, p value of 3.44 × 10−4). (***p value < 0.001).
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 3: Rare predicted pathogenic mutations
Exome: Other
Images
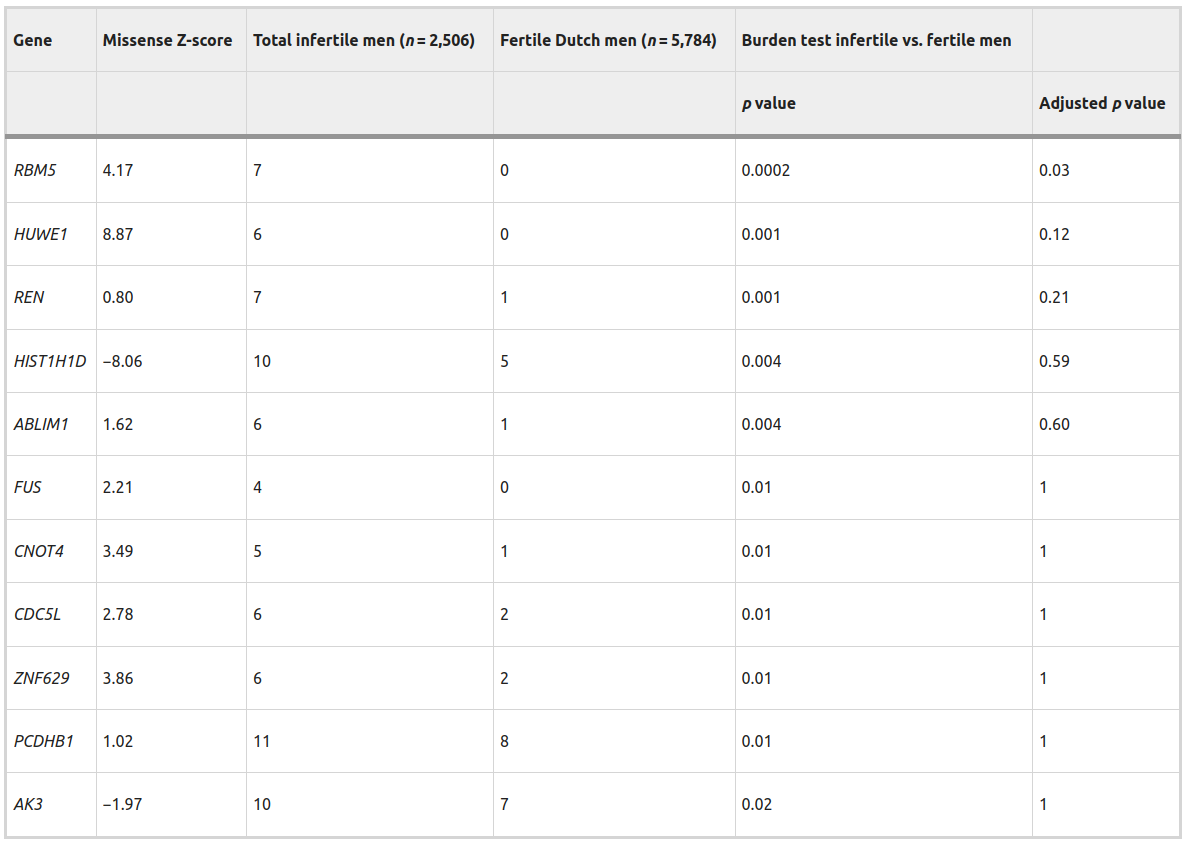
Table 1: Rare pathogenic missense mutations in exome data from various cohorts of infertile men and fertile control cohorts.
Genes affected by a rare missense DNM were investigated in additional cohorts of infertile patients and a cohort of verified fertile men to identify other individuals carrying rare missense mutations. A burden test was used to compare the total number of predicted pathogenic missense mutations observed in the infertile vs. fertile men. A two-tailed Fisher’s Exact test was performed with and without Bonferroni correction applied to adjust p values for multiple testing of all 152 genes of interest.
Licensed under: https://creativecommons.org/licenses/by/4.0/
