The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty
Guo J, Nie X, Giebler M, Mlcochova H, Wang Y, Grow EJ; DonorConnect; Kim R, Tharmalingam M, Matilionyte G, Lindskog C, Carrell DT, Mitchell RT, Goriely A, Hotaling JM, Cairns BR, 09.01.2020
Abstract
The human testis undergoes dramatic developmental and structural changes during puberty, including proliferation and maturation of somatic niche cells, and the onset of spermatogenesis. To characterize this understudied process, we profiled and analyzed single-cell transcriptomes of ∼10,000 testicular cells from four boys spanning puberty and compared them to those of infants and adults. During puberty, undifferentiated spermatogonia sequentially expand and differentiate prior to the initiation of gametogenesis. Notably, we identify a common pre-pubertal progenitor for Leydig and myoid cells and delineate candidate factors controlling pubertal differentiation. Furthermore, pre-pubertal Sertoli cells exhibit two distinct transcriptional states differing in metabolic profiles before converging to an alternative single mature population during puberty. Roles for testosterone in Sertoli cell maturation, antimicrobial peptide secretion, and spermatogonial differentiation are further highlighted through single-cell analysis of testosterone-suppressed transfemale testes. Taken together, our transcriptional atlas of the developing human testis provides multiple insights into developmental changes and key factors accompanying male puberty.
Guo J, Nie X, Giebler M, Mlcochova H, Wang Y, Grow EJ; DonorConnect; Kim R, Tharmalingam M, Matilionyte G, Lindskog C, Carrell DT, Mitchell RT, Goriely A, Hotaling JM, Cairns BR. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell. 2020 Feb 6;26(2):262-276.e4. doi: 10.1016/j.stem.2019.12.005. Epub 2020 Jan 9. PMID: 31928944; PMCID: PMC7298616.
Publication: https://doi.org/10.1016/j.stem.2019.12.005
 Disclaimer
Disclaimer
The publication The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty by Guo J, Nie X, Giebler M, Mlcochova H, Wang Y, Grow EJ; DonorConnect; Kim R, Tharmalingam M, Matilionyte G, Lindskog C, Carrell DT, Mitchell RT, Goriely A, Hotaling JM, Cairns BR is published under an open access license: http://creativecommons.org/licenses/by/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Single-cell transcriptome profiling of the human juvenile testis
Transcriptome: Single-cell RNA-Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | infant (1 year), juvenile (7, 11, 13, and 14 years), adult (25 years) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 6 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000586: germ cell | The reproductive cell in multicellular organisms. | Human | |||
| CL_0000020: spermatogonium | An euploid male germ cell of an early stage of spermatogenesis. | Human | |||
| CL_0000017: spermatocyte | A male germ cell that develops from spermatogonia. The euploid primary spermatocytes undergo meiosis and give rise to the haploid secondary spermatocytes which in turn give rise to spermatids. | Human | |||
| CL_0000018: spermatid | A male germ cell that develops from the haploid secondary spermatocytes. Without further division, spermatids undergo structural changes and give rise to spermatozoa. | Human | |||
| CL_0000216: Sertoli cell | A supporting cell projecting inward from the basement membrane of seminiferous tubules. They surround and nourish the developing male germ cells and secrete androgen binding protein. Their tight junctions with the spermatogonia and spermatocytes provide a blood-testis barrier. | Human | |||
| CL_0000178: Leydig cell | A Leydig cell is a testosterone-secreting cell in the interstitial area, between the seminiferous tubules, in the testis. | Human | |||
| CL_0002481: peritubular myoid cell | The flattened smooth myoepithelial cells of mesodermal origin that lie just outside the basal lamina of the seminiferous tubule. | Human | |||
| CL_0000235: macrophage | A mononuclear phagocyte present in variety of tissues, typically differentiated from monocytes, capable of phagocytosing a variety of extracellular particulate material, including immune complexes, microorganisms, and dead cells. | Human | |||
| CL_0000115: endothelial cell | An endothelial cell comprises the outermost layer or lining of anatomical structures and can be squamous or cuboidal. In mammals, endothelial cell has vimentin filaments and is derived from the mesoderm. | Human | |||
| CL_0000192: smooth muscle cell | A non-striated, elongated, spindle-shaped cell found lining the digestive tract, uterus, and blood vessels. They develop from specialized myoblasts (smooth muscle myoblast). | Human |
Images
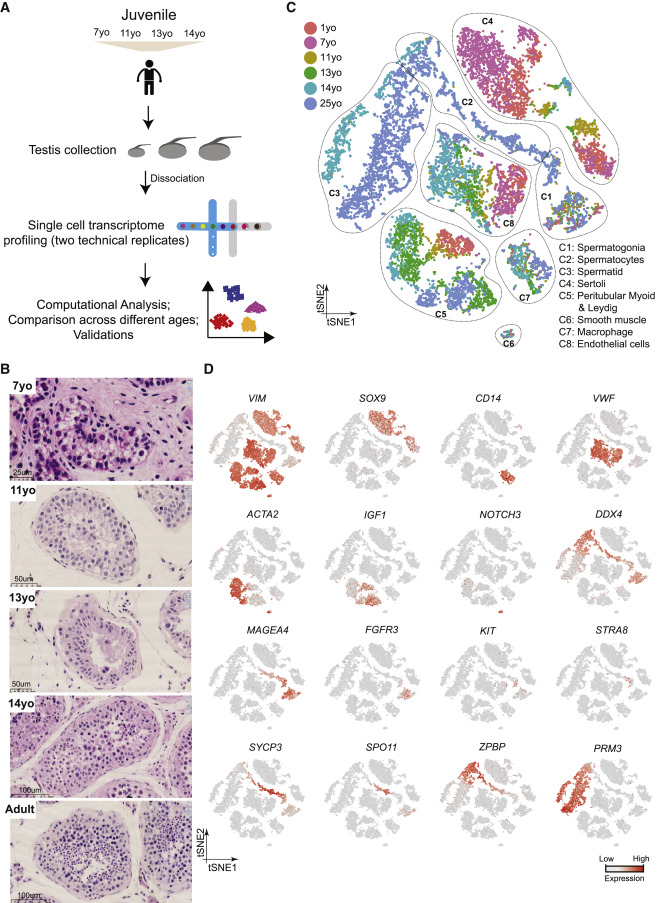
Figure 1: Single-Cell Transcriptome Profiling of the Human Juvenile Testis
(A) Schematic illustration of the experimental workflow. (B) H&E (hematoxylin and eosin) staining of the different juvenile testes analyzed in this study illustrates the typical macroscopic testicular changes in morphology observed during puberty. (C) tSNE and clustering analysis of single-cell transcriptome data from juvenile human testes (n = 7,680), combined with previous datasets of infant and adult scRNA-seq (Guo et al., 2018). Each dot represents a single cell and is colored according to its donor of origin. tSNE, t-distributed stochastic neighbor embedding. (D) Expression patterns of selected markers projected on the tSNE plot (Figure 1C). For each cell cluster, one cell marker is shown in the main figure accompanied by a gallery of further markers in Figures S1D and S1E. The two top rows show somatic/niche cell markers; representative germ cell markers are shown in the two bottom rows.
Licensed under: http://creativecommons.org/licenses/by/4.0/
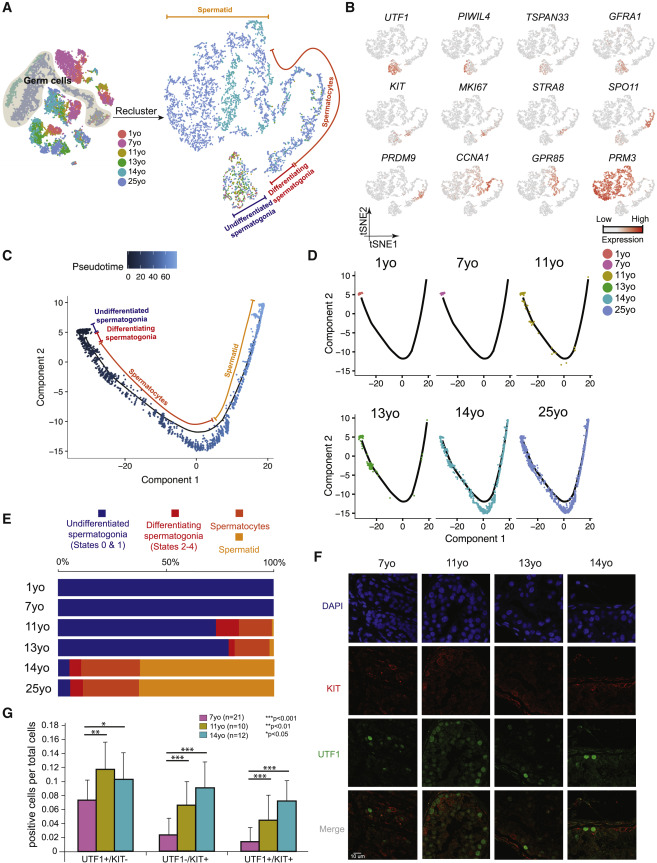
Figure 2: Distinct Phases of Spermatogonial Proliferation and Differentiation during Human Puberty
(A) Focused analysis (tSNE and clustering) of the germ cells (clusters C1, C2, and C3 from Figure 1C) reveals developmental progression of spermatogenesis during puberty. Cells are colored based on the ages/donors of origin. (B) Expression patterns of known spermatogenic markers projected onto the tSNE plot from Figure 2A. (C) Pseudotime trajectory (Monocle analysis) of the germ cells. Cells are colored based according to the predicted pseudotime. (D) Deconvolution of the Monocle pseudotime plot according to ages/donors of origin. (E) Relative proportion of the single cells at different spermatogenic stages in the samples analyzed. (F) Protein co-immunofluorescence for two spermatogonial cell markers: UTF1 (States 0–1) and KIT (States 2–4) in the 4 analyzed samples. See Figure S2D for a wider field of view. (G) Quantification of UTF1+ and/or KIT+ spermatogonia at different ages. The data shown are means ± SD of independent tubules. The p value was calculated via Student’s t test.
Licensed under: http://creativecommons.org/licenses/by/4.0/
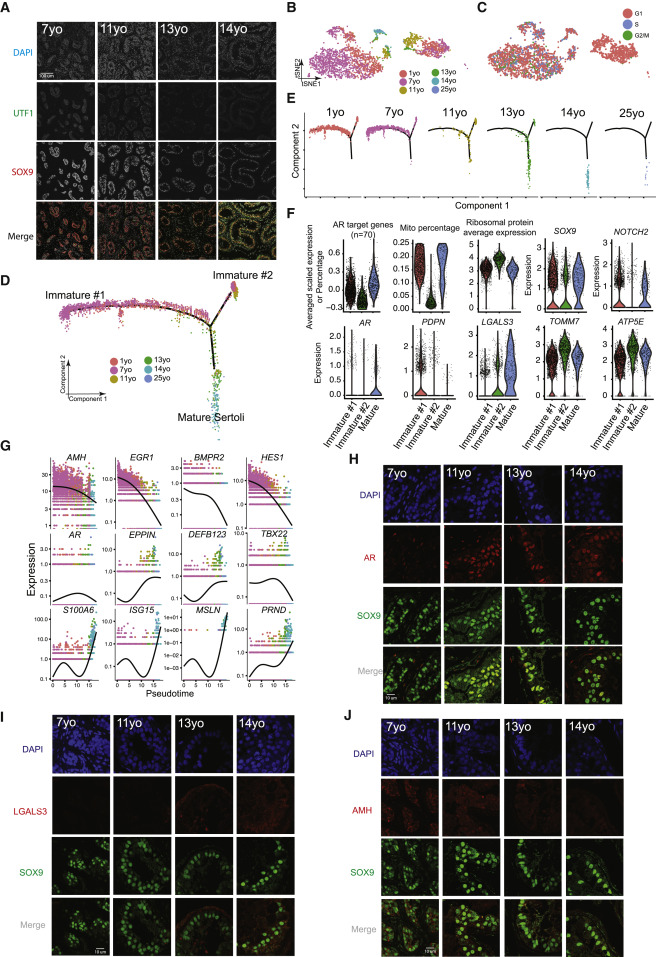
Figure 3: Identification of Two Sertoli States in the Pre-pubertal Testis
(A) Immunolocalization of germ cells and Sertoli cells in the analyzed samples (7–14 years old) illustrated by co- staining with UTF1 and SOX9. (B) Focused analysis (tSNE and reclustering) of Sertoli cells (cluster C4 from Figure 1C), with cells colored by ages/donors. (C) Focused analysis (tSNE and reclustering) of Sertoli cells, with cells colored according to their cell-cycle phase (G1/S/G2). (D) Pseudotime trajectory (via Monocle) of Sertoli cells revealed two distinct early (immature) cellular states that progressively converge along the pseudotime. (E) Deconvolution of the Monocle pseudotime plot according to ages/donors of origin. (F) Selected key genes/programs showing differential expression in the distinct Sertoli states. (G) Expression patterns of representative dynamic genes during Sertoli cell maturation, as predicted by pseudotime. (H) Immunofluorescent co-staining for SOX9 and AR at different ages (7–14 years old) shows an increase in AR expression during juvenile development. (I) Immunofluorescent co-staining for SOX9 and LGALS3 at different ages (7–14 years old) supports the progressive maturation of the Sertoli cells shown by pseudotime trajectory. (J) Immunofluorescent co-staining for SOX9 and AMH at different ages (7–14 years old) supports the gradual maturation of Sertoli cells over time shown by pseudotime trajectory.
Licensed under: http://creativecommons.org/licenses/by/4.0/
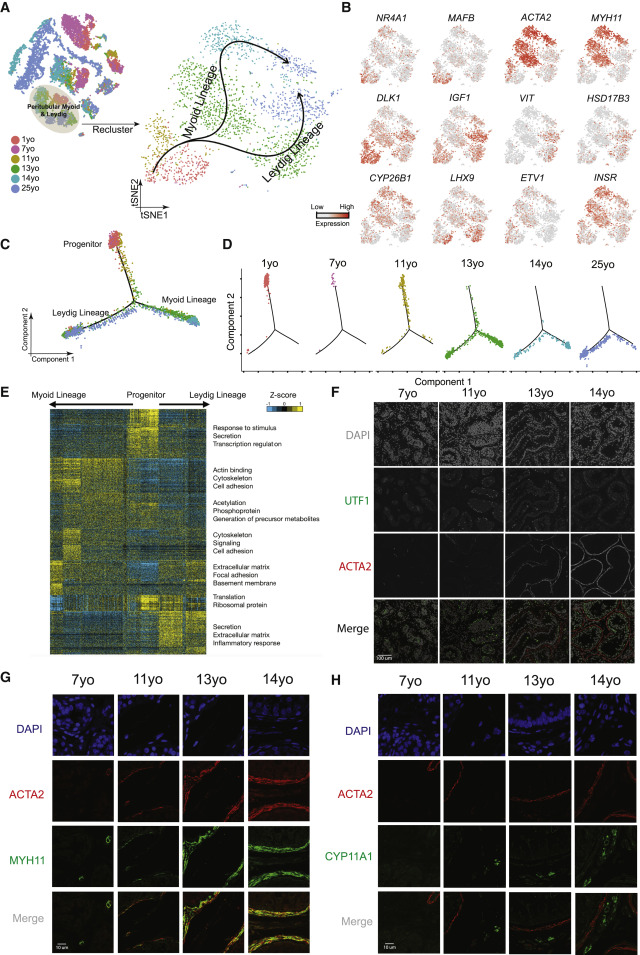
Figure 4: A Common Progenitor for Leydig and Peritubular Myoid Cells
(A) Focused analysis (tSNE and clustering) of the Leydig and peritubular myoid cells (cluster C5 from Figure 1C), with cells colored according to ages/donors of origin. The predicted developmental lineages are represented by the arrows. (B) Expression patterns of key representative markers projected onto the tSNE plot from Figure 4A. (C) Pseudotime trajectory (via Monocle) of the C5 cluster predicts a common early pre-pubertal progenitor state and two distinct developmental trajectories for the Leydig and myoid lineages during puberty. (D) Deconvolution of the Monocle pseudotime plot according to the ages/donors of origin. (E) K-means clustering of genes exhibiting differential expression (n = 1,005) along the Leydig and myoid cell lineages. Each row represents a gene, and each column represents a single cell, with columns/cells placed in pseudotime order defined in Figure 4C. Differential gene expression levels utilize a Z score as defined by the color key; associated GO terms (using DAVID v6.7) are given on the right of the corresponding gene clusters. (F) Protein co-immunofluorescence for UTF1 and the peritubular myoid cell marker ACTA2 in the analyzed samples (7–14 years old) revealed that the myoid lineage (ACTA2+) is progressively specified during puberty. (G) Protein co-immunofluorescence for two known myoid cell markers, ACTA2 and MYH11, at different ages (7–14 years old). (H) Immunofluorescent co-staining for ACTA2 and CYP11A1 (Leydig cell marker) shows the progressive expression of them during juvenile development.
Licensed under: http://creativecommons.org/licenses/by/4.0/
Data set 2: Single-cell profiling of testes from T-suppressed transfemales
Transcriptome: Single-cell RNA-Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | Adult, 50 years old (T-suppressed) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 1 |
| BTO_0001363: testis | Adult, 26 years old (T-suppressed) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 1 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000020: spermatogonium | An euploid male germ cell of an early stage of spermatogenesis. | Human | |||
| CL_0000017: spermatocyte | A male germ cell that develops from spermatogonia. The euploid primary spermatocytes undergo meiosis and give rise to the haploid secondary spermatocytes which in turn give rise to spermatids. | Human | |||
| CL_0000018: spermatid | A male germ cell that develops from the haploid secondary spermatocytes. Without further division, spermatids undergo structural changes and give rise to spermatozoa. | Human | |||
| CL_0000192: smooth muscle cell | A non-striated, elongated, spindle-shaped cell found lining the digestive tract, uterus, and blood vessels. They develop from specialized myoblasts (smooth muscle myoblast). | Human | |||
| CL_0000235: macrophage | A mononuclear phagocyte present in variety of tissues, typically differentiated from monocytes, capable of phagocytosing a variety of extracellular particulate material, including immune complexes, microorganisms, and dead cells. | Human | |||
| CL_0002481: peritubular myoid cell | The flattened smooth myoepithelial cells of mesodermal origin that lie just outside the basal lamina of the seminiferous tubule. | Human | |||
| CL_0000115: endothelial cell | An endothelial cell comprises the outermost layer or lining of anatomical structures and can be squamous or cuboidal. In mammals, endothelial cell has vimentin filaments and is derived from the mesoderm. | Human | |||
| CL_0000178: Leydig cell | A Leydig cell is a testosterone-secreting cell in the interstitial area, between the seminiferous tubules, in the testis. | Human | |||
| CL_0000216: Sertoli cell | A supporting cell projecting inward from the basement membrane of seminiferous tubules. They surround and nourish the developing male germ cells and secrete androgen binding protein. Their tight junctions with the spermatogonia and spermatocytes provide a blood-testis barrier. | Human | |||
| CL_0000910: cytotoxic T cell | A mature T cell that differentiated and acquired cytotoxic function with the phenotype perforin-positive and granzyme-B positive. | Human |
Images
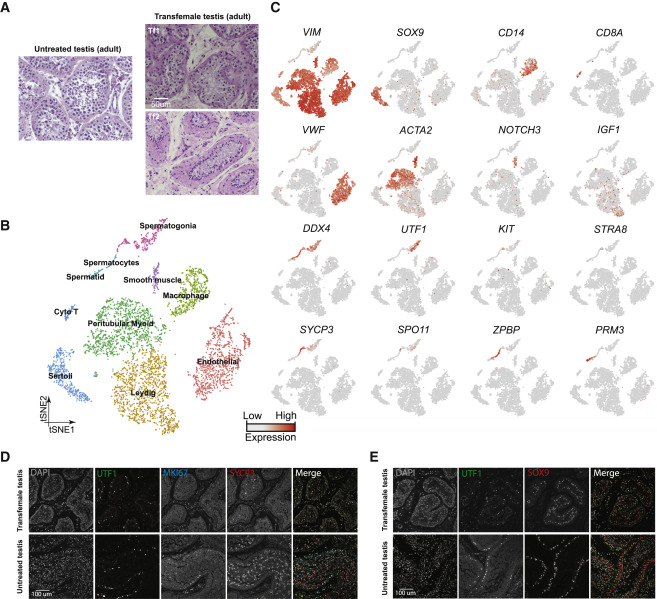
Figure 5: Single-Cell Transcriptome Profiling of Testes from T-Suppressed Transfemales
(A) H&E staining of the adult untreated (25 years old) and T-suppressed (Tf1 and Tf2) testicular sections. (B) tSNE and clustering analysis of single-cell transcriptome data from two transfemale testes (n = 5,179). (C) Expression patterns of selected markers projected on the tSNE plot. Top two rows are somatic/niche cell markers; bottom two rows are representative germ cell markers. (D) Examination of germ cell compositions in T-suppressed (Tf1 as an example) and untreated (25 years old) testis by protein immunostaining of three germ cell markers. (E) Immunolocalization of germ cells and Sertoli cells in T-suppressed (Tf1 as an example) and untreated (25 years old) testis by staining for UTF1 and SOX9.
Licensed under: http://creativecommons.org/licenses/by/4.0/
Data set 3: Testosterone promotes Sertoli and germ cell development in vivo
Transcriptome: Single-cell RNA-Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | untreated -> 1 infant (1 year), 4 juveniles (7, 11, 13, and 14 years), 1 adult (25 years) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 6 |
| BTO_0001363: testis | T-suppressed transfemales (50 and 26 years old) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 2 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000910: cytotoxic T cell | A mature T cell that differentiated and acquired cytotoxic function with the phenotype perforin-positive and granzyme-B positive. | Human | |||
| CL_0000235: macrophage | A mononuclear phagocyte present in variety of tissues, typically differentiated from monocytes, capable of phagocytosing a variety of extracellular particulate material, including immune complexes, microorganisms, and dead cells. | Human | |||
| CL_0000115: endothelial cell | An endothelial cell comprises the outermost layer or lining of anatomical structures and can be squamous or cuboidal. In mammals, endothelial cell has vimentin filaments and is derived from the mesoderm. | Human | |||
| CL_0000216: Sertoli cell | A supporting cell projecting inward from the basement membrane of seminiferous tubules. They surround and nourish the developing male germ cells and secrete androgen binding protein. Their tight junctions with the spermatogonia and spermatocytes provide a blood-testis barrier. | Human | |||
| CL_0000018: spermatid | A male germ cell that develops from the haploid secondary spermatocytes. Without further division, spermatids undergo structural changes and give rise to spermatozoa. | Human | |||
| CL_0000017: spermatocyte | A male germ cell that develops from spermatogonia. The euploid primary spermatocytes undergo meiosis and give rise to the haploid secondary spermatocytes which in turn give rise to spermatids. | Human | |||
| CL_0000020: spermatogonium | An euploid male germ cell of an early stage of spermatogenesis. | Human | |||
| CL_0002481: peritubular myoid cell | The flattened smooth myoepithelial cells of mesodermal origin that lie just outside the basal lamina of the seminiferous tubule. | Human | |||
| CL_0000178: Leydig cell | A Leydig cell is a testosterone-secreting cell in the interstitial area, between the seminiferous tubules, in the testis. | Human | |||
| CL_0000192: smooth muscle cell | A non-striated, elongated, spindle-shaped cell found lining the digestive tract, uterus, and blood vessels. They develop from specialized myoblasts (smooth muscle myoblast). | Human |
Images
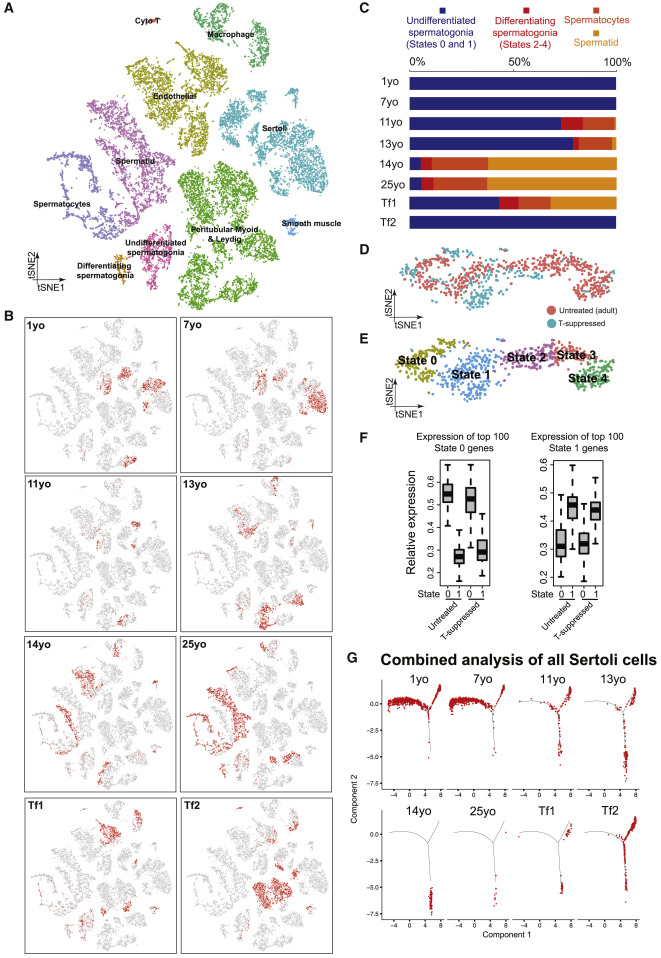
Figure 6: Testosterone Promotes Sertoli and Germ Cell Development In Vivo
(A) tSNE and clustering analysis of combined single-cell transcriptome data from T-suppressed (Tf1 and Tf2) and untreated (from infancy through puberty to adult) testis, with cells colored based on their identities. See Figure S6D for the markers used to assign the cell identities. (B) Partitioning the combined tSNE analysis in Figure 6A based on the ages/donors of origin, with cells from each donor highlighted in red separately in different boxes. (C) Relative proportion of cells at different spermatogenic stages in untreated testes (different ages) or T-suppressed testes. (D and E) Comparison of spermatogonia from untreated adult (5 States as defined in Guo et al., 2018) and T-suppressed testes via tSNE analysis, with cells colored according to their T treatment (D) or spermatogonial states (E). (F) Expression levels of spermatogonial State 0 genes (left) or State 1 genes (right) in untreated or T suppressed. Neither State 0, nor State 1 cells from untreated and T-suppressed testes showed statistically significant differences in gene expression via Wilcoxon test (p value = 0.528 [left] and 0.065 [right]). (G) Comparison of Sertoli cells profile in all samples from T-suppressed (Tf1 and Tf2) and untreated (from infancy through puberty to adult) testes using focused pseudotime analysis (via Monocle).
Licensed under: http://creativecommons.org/licenses/by/4.0/
