The inherited methylome landscape is directly altered with paternal aging and associated with offspring neurodevelopmental disorders
Denomme MM, Haywood ME, Parks JC, Schoolcraft WB, Katz-Jaffe MG, 01.07.2020
Abstract
Paternal aging and the prevalence of neurodevelopmental disorders in offspring are well documented. Yet, the underlying mechanism and the mode of inheritance have not been conclusively established. Advancing paternal age is a subtle and varying phenotype. As such, it is likely that a threshold for cumulative risk may exist that, if surpassed, culminates in a predisposition to disease and ultimately an observed phenotype in offspring. Epigenetic regulation provides a plausible explanation for the nongenetic paternal transmission of disease susceptibility. With the use of whole-genome methylation sequencing, the data described herein substantiate an increasingly compromised DNA methylation profile as sperm ages and, for the first time, also demonstrate a generational correlation in sperm and blastocyst of an altered methylome associated with advanced paternal age. Methylation alterations are not randomly distributed across the genome, but appear clustered at certain chromosomal locations, and significantly colocalize with regions of nucleosome retention. Genes associated with autism spectrum disorder, schizophrenia, and bipolar disorder are significantly enriched with causative methylation aberrations in both sperm and embryos from aged fathers. The long-term health burden and societal economic impact of these conditions are substantial and will continue with increasingly prevalent diagnosis. This work provides a mechanistic link between the paternal age effect and offspring neurodevelopmental disorders leading to a better understanding of causation and investigation into potential future therapy.
Denomme MM, Haywood ME, Parks JC, Schoolcraft WB, Katz-Jaffe MG. The inherited methylome landscape is directly altered with paternal aging and associated with offspring neurodevelopmental disorders. Aging Cell. 2020 Aug;19(8):e13178. doi: 10.1111/acel.13178. Epub 2020 Jul 1. PMID: 32610362; PMCID: PMC7431824.
Publication: https://doi.org/10.1111/acel.13178
 Disclaimer
Disclaimer
The publication The inherited methylome landscape is directly altered with paternal aging and associated with offspring neurodevelopmental disorders by Denomme MM, Haywood ME, Parks JC, Schoolcraft WB, Katz-Jaffe MG is published under an open access license: https://creativecommons.org/licenses/by/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Sperm methylomes
Methylome: Reduced-Representation Bisulfite Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | Adult | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 12 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm | A mature male germ cell that develops from a spermatid. | Human |
Images
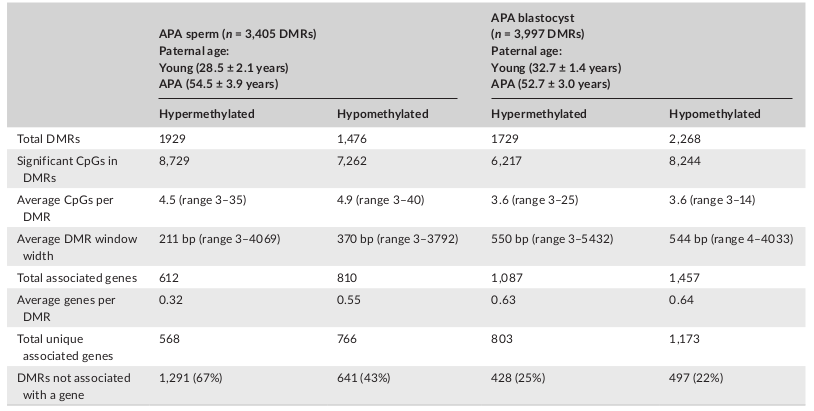
Table 1: APA sperm and blastocyst DMRs
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 2: Blastocyst methylomes
Methylome: Whole Genome Bisulfite Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001424: uterus | An organ of the female mammal for containing and usually for nourishing the young during development previous to birth. | Human | 12 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| UBERON_0000358: blastocyst | The mammalian blastocyst is a hollow ball of cells containing two cell types, the inner cell mass and the trophectoderm. | Human |
Images
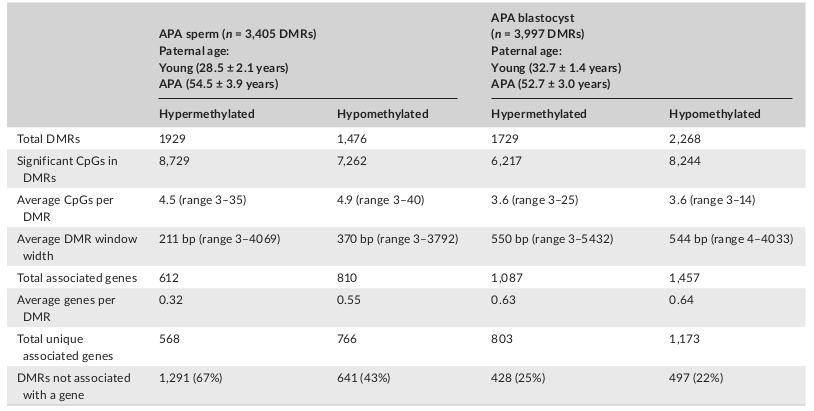
Table 1: APA sperm and blastocyst DMRs
Licensed under: https://creativecommons.org/licenses/by/4.0/
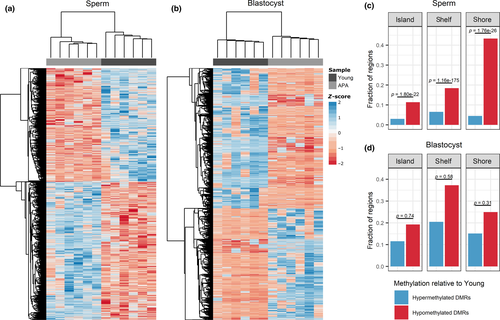
Figure 1: Significant DMR-associated CpG sites and the fraction of DMRs localized to CpG islands, shelves, and shores
(a–b) Heat map representation of the hierarchical clustering of significant (p ≤ .05) DMR-associated CpG sites in a) sperm (n = 15,991 CpGs) and b) blastocyst (n = 14,461 CpGs), where a positive z-score corresponds to hypermethylation and a negative z-score corresponds to hypomethylation relative to the mean. Samples in both sperm and blastocyst cluster into two distinct groups by young and APA samples. (c–d) The proportion of DMRs associated with CpG islands, shelves, and shores in c) sperm and d) blastocyst was tallied for hypermethylated and hypomethylated DMRs in APA relative to young. Hypomethylated CpG regions were significantly enriched in sperm but not blastocyst
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 3: Targeted bisulfite pyrosequencing for methylation validation
Methylome: Targeted Bisulfite Pyrosequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | Adult | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 24 |
| BTO_0001424: uterus | Adult | An organ of the female mammal for containing and usually for nourishing the young during development previous to birth. | Human | 24 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm | A mature male germ cell that develops from a spermatid. | Human | |||
| UBERON_0000358: blastocyst | The mammalian blastocyst is a hollow ball of cells containing two cell types, the inner cell mass and the trophectoderm. | Human |
Images
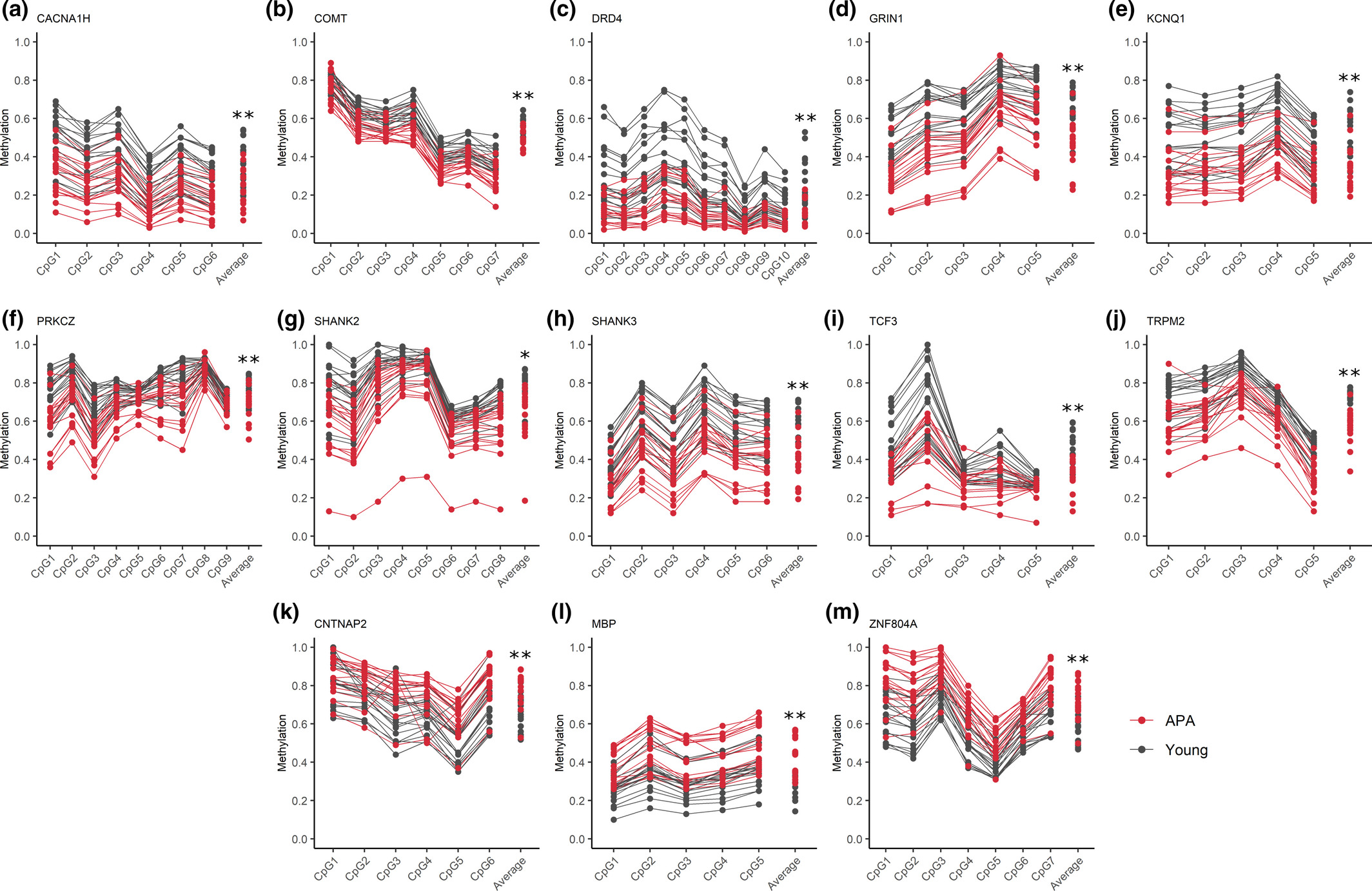
Figure 2: Sperm DNA methylation validation results
Each plot represents pyrosequencing results for selected validation genes at distinct CpG sites within a significant DMR. CpG sites on the x-axis are followed by the average percent methylation across all CpGs for young (gray) and APA (red) individuals. Each line represents results for individual sperm samples (n = 18 young and n = 18 APA sperm samples). Significant hypomethylation in APA relative to young is demonstrated in plots (A-J), and significant hypermethylation in APA relative to young is demonstrated in plots (K-M); *p ≤ .01, **p ≤ .001
Licensed under: https://creativecommons.org/licenses/by/4.0/
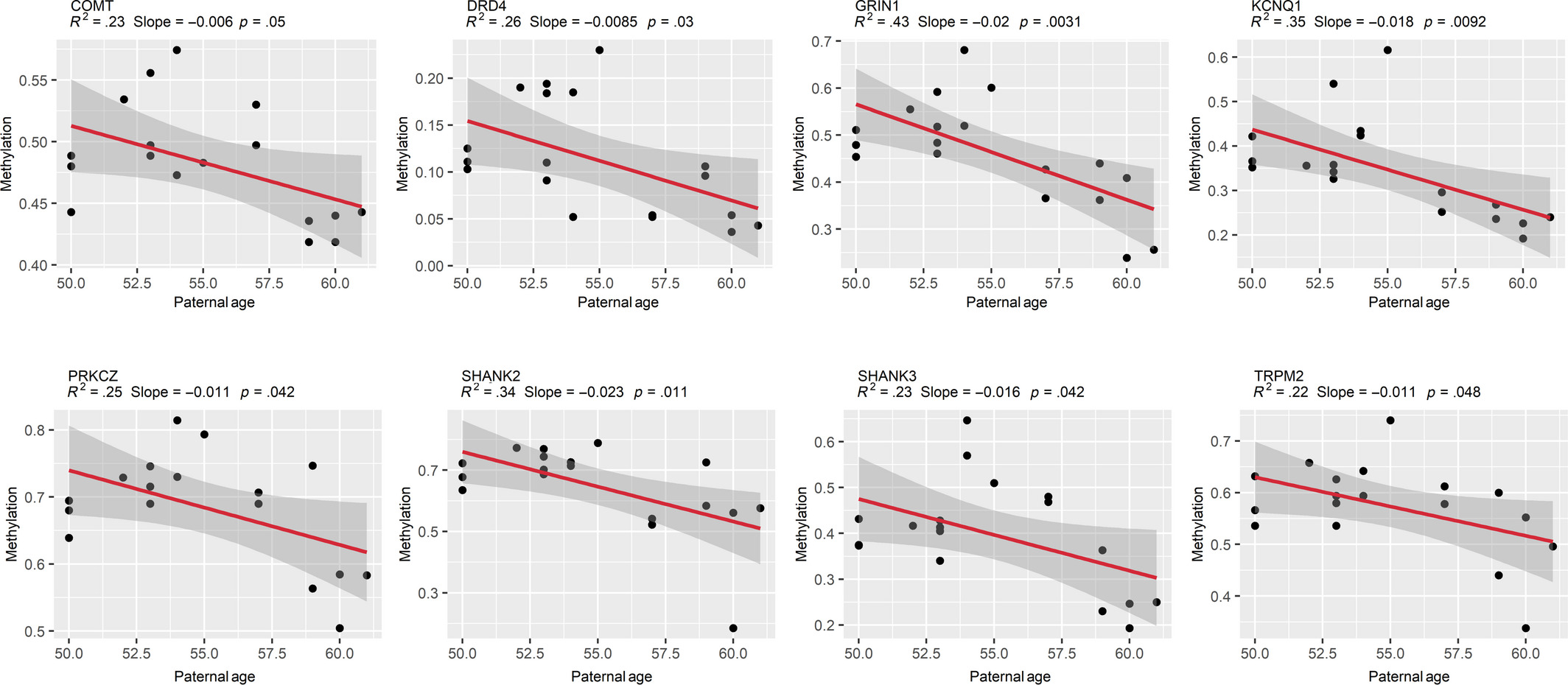
Figure 3: Sperm DNA methylation change by paternal age
Linear regression models demonstrate a significant negative association between sperm DNA methylation and paternal age in APA fathers (≥50 years; n = 18 APA sperm samples). Models were fitted using the lm() function in R with default arguments, and those with p ≤ .05 were considered significant. R-squared, slope, and p-values are displayed above each plot, and shaded gray areas around each red regression line represent a 95% confidence interval
Licensed under: https://creativecommons.org/licenses/by/4.0/
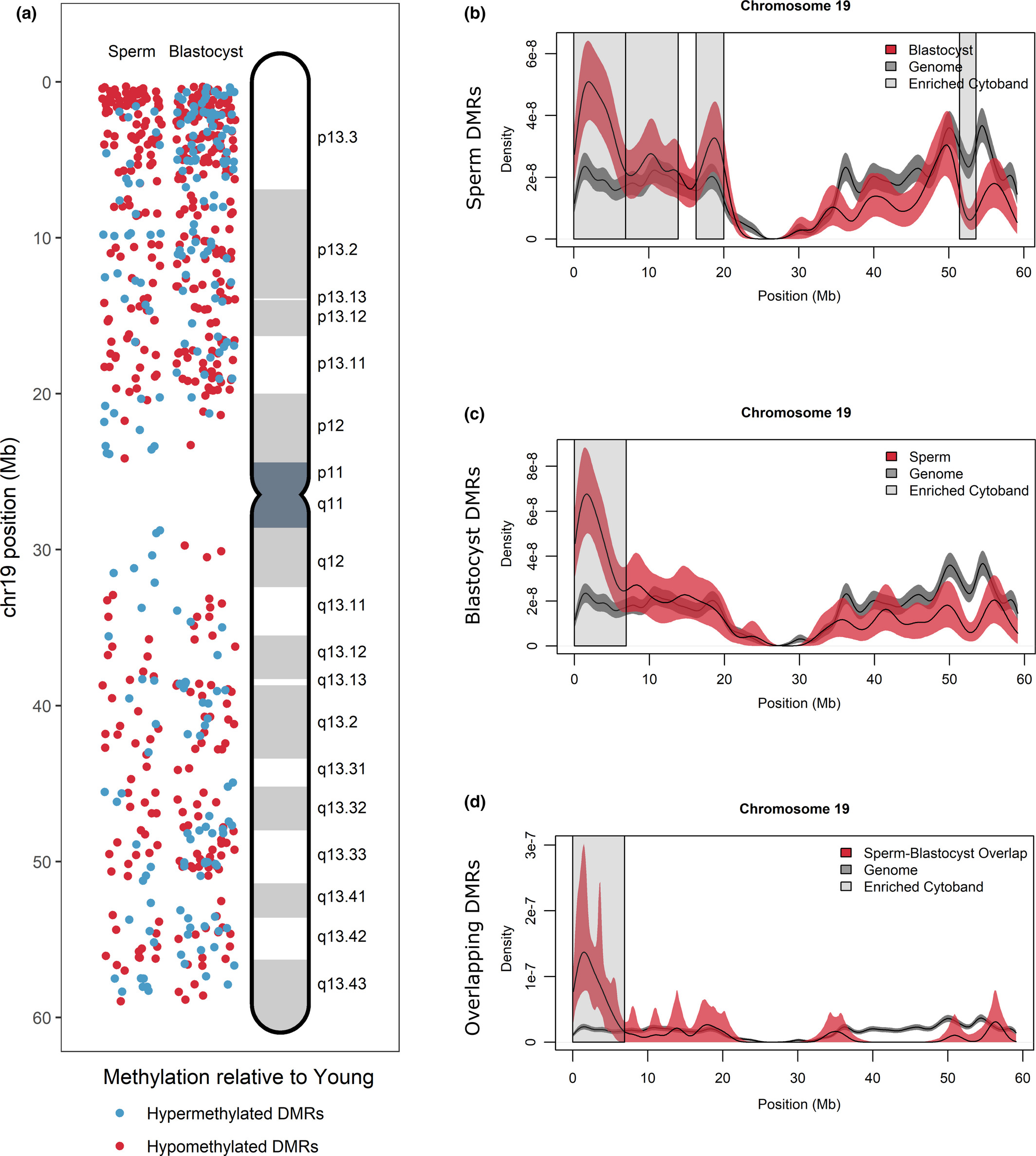
Figure 4: DMR localization on Chromosome 19
(a) DMRs on chromosome 19 were localized for sperm (left panel) and blastocyst (right panel) according to hypermethylated DMRs (blue) and hypomethylated DMRs (red) in APA relative to young. Increased DMR clustering is observed for cytoband 19p13.3. (b–d) All genes located on chromosome 19 (gray lines) were compared with DMR-associated genes (red lines) in (b) sperm, (c) blastocyst, and (d) overlapping sperm blastocyst using kernel density estimates. Shaded areas around the lines signify the 95% confidence interval. Cytobands where the density of genes was significantly different between DMR-associated genes and the genome are highlighted with gray boxes and include cytoband 19p13.3 for all comparisons
Licensed under: https://creativecommons.org/licenses/by/4.0/

Table 2: DMR-associated genes in autistic brains compared with APA sperm and APA blastocyst
Licensed under: https://creativecommons.org/licenses/by/4.0/
