DNMT3A-dependent DNA methylation is required for spermatogonial stem cells to commit to spermatogenesis
Dura M, Teissandier A, Armand M, Barau J, Lapoujade C, Fouchet P, Bonneville L, Schulz M, Weber M, Baudrin LG, Lameiras S, Bourc'his D, 11.04.2022
Abstract
DNA methylation plays a critical role in spermatogenesis, as evidenced by the male sterility of DNA methyltransferase (DNMT) mutant mice. Here, we report a division of labor in the establishment of the methylation landscape of male germ cells and its functions in spermatogenesis. Although DNMT3C is essential for preventing retrotransposons from interfering with meiosis, DNMT3A broadly methylates the genome (with the exception of DNMT3C-dependent retrotransposons) and controls spermatogonial stem cell (SSC) plasticity. By reconstructing developmental trajectories through single-cell RNA sequencing and profiling chromatin states, we found that Dnmt3A mutant SSCs can only self-renew and no longer differentiate in association with spurious enhancer activation that enforces an irreversible stem cell gene program. Our findings therefore highlight a key function of DNA methylation in male fertility: the epigenetic programming of SSC commitment to differentiation and lifelong spermatogenesis supply.
Dura M, Teissandier A, Armand M, Barau J, Lapoujade C, Fouchet P, Bonneville L, Schulz M, Weber M, Baudrin LG, Lameiras S, Bourc'his D. DNMT3A-dependent DNA methylation is required for spermatogonial stem cells to commit to spermatogenesis. Nat Genet. 2022 Apr;54(4):469-480. doi: 10.1038/s41588-022-01040-z. Epub 2022 Apr 11. PMID: 35410378.
Publication: https://doi.org/10.1038/s41588-022-01040-z
 Disclaimer
Disclaimer
The publication DNMT3A-dependent DNA methylation is required for spermatogonial stem cells to commit to spermatogenesis by Dura M, Teissandier A, Armand M, Barau J, Lapoujade C, Fouchet P, Bonneville L, Schulz M, Weber M, Baudrin LG, Lameiras S, Bourc'his D is published under an open access license: https://creativecommons.org/licenses/by/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: DNMT3A broadly methylates the genome in fetal male germ cells
Methylome: Whole Genome Bisulfite Sequencing
Species
| Species |
|---|
| Mouse |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | embryonic day 18.5 | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Mouse |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0002238: gonocyte | A primordial germ cell that is destined to become a male germ cell. | Mouse |
Images
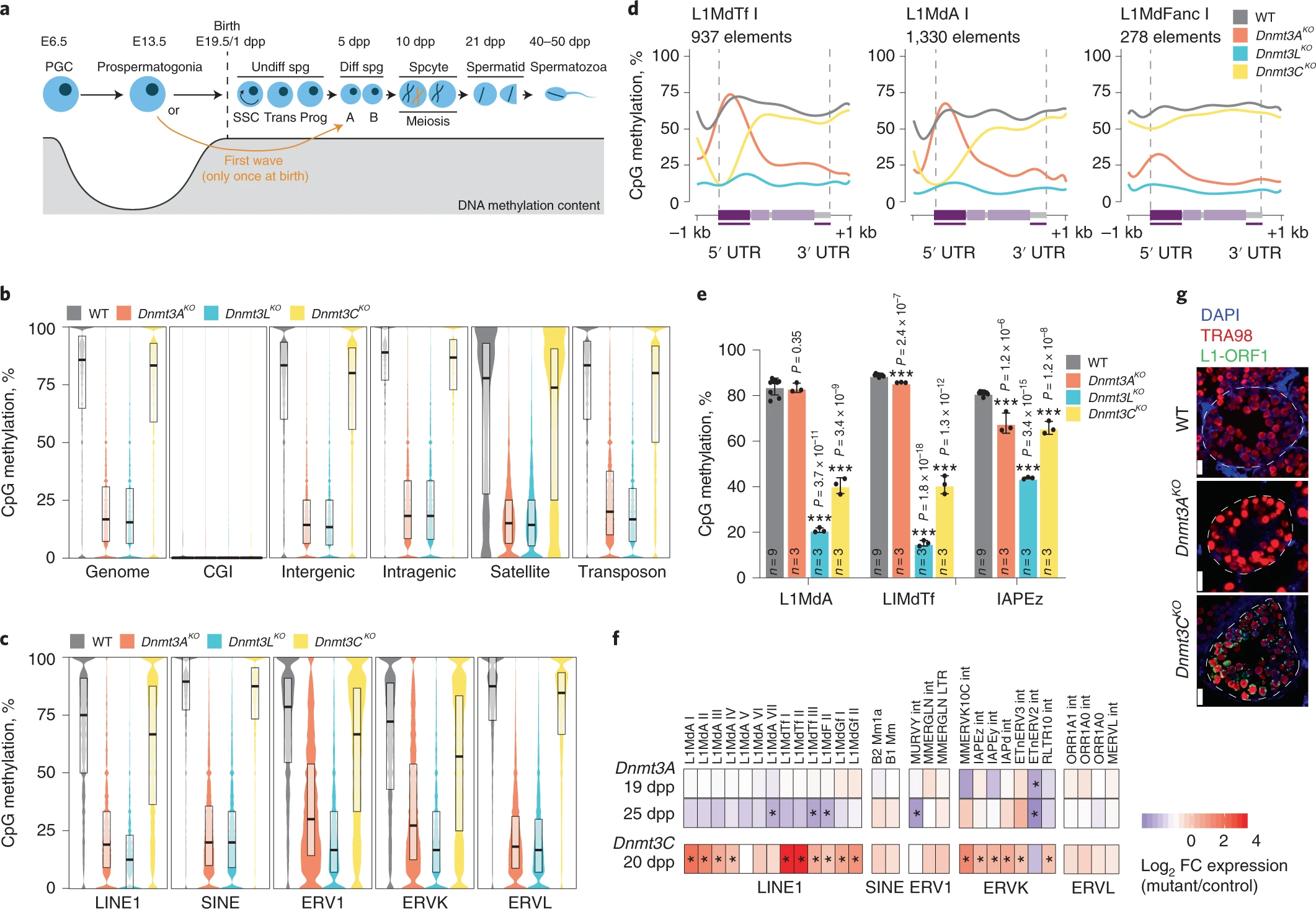
Figure 1: DNMT3A broadly methylates the male germ cell genome but is dispensable for retrotransposon silencing
a, Schematic representation of developmental and DNA methylation dynamics of the male germline in the mouse. Postnatal ages indicate predominant germ cell types present in the testis during the first wave of spermatogenesis. PGC, primordial germ cell; Spg, spermatogonia; Trans, transitory; Prog, progenitors. b,c, Violin plot representation of percentage of methylated CpGs across the genome and different genomic compartments (b) and over retrotransposon families (c) in WT (gray), Dnmt3AKO (orange), Dnmt3LKO (blue) and Dnmt3CKO (yellow) fetal germ cells (E18.5), as determined by WGBS. Black horizontal bars represent the median, and upper and lower hinges correspond to 75% and 25% quantile. Each sample is a pool of three embryos of the same genotype. CGI, CpG islands. d, Metaplots of DNA methylation levels over uniquely assigned full-length elements (>5 kb) from three L1 families. e, CpG methylation levels assessed by bisulfite pyrosequencing of the promoters of indicated retrotransposon families in 10 dpp sorted germ cells (EpCAM positive, β2-microglobulin negative). Data are mean ± s.d. (black bar) from biological replicates, and black dots represent biological replicates; n = number of animals (one-sided Student’s t test over WT: ***P < 0.0005). f, RNA-seq heatmap shows log2 fold change (FC) in retrotransposon mRNA levels in Dnmt3AKO versus WT littermates at 19 and 25 dpp (top and middle rows), and previously reported changes in Dnmt3C mutants versus heterozygous littermates at 20 dpp (bottom row)12. Annotations are from RepeatMasker. Two biological replicates were sequenced per age and genotype. g, L1-ORF1 immunostaining on testis sections at 19 dpp in WT, Dnmt3AKO and Dnmt3CKO germ cells (TRA98 positive). White dotted lines show tubule delineation. Purple, Red and Green text refer to the color of the fluorsecent staining on the microscopy image. Scale bars, 20 μm.
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 2: SSCs cannot exit the stem cell state without DNMT3A-dependent DNA methylation
Transcriptome: Single-cell RNA-Sequencing
Species
| Species |
|---|
| Mouse |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Mouse | 8 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000586: germ cell | The reproductive cell in multicellular organisms. | ||||
| CL_0000020: spermatogonium | An euploid male germ cell of an early stage of spermatogenesis. | Mouse | |||
| CL_0000016: male germ line stem cell | A stem cell that is the precursor of male gametes. | Mouse |
Images
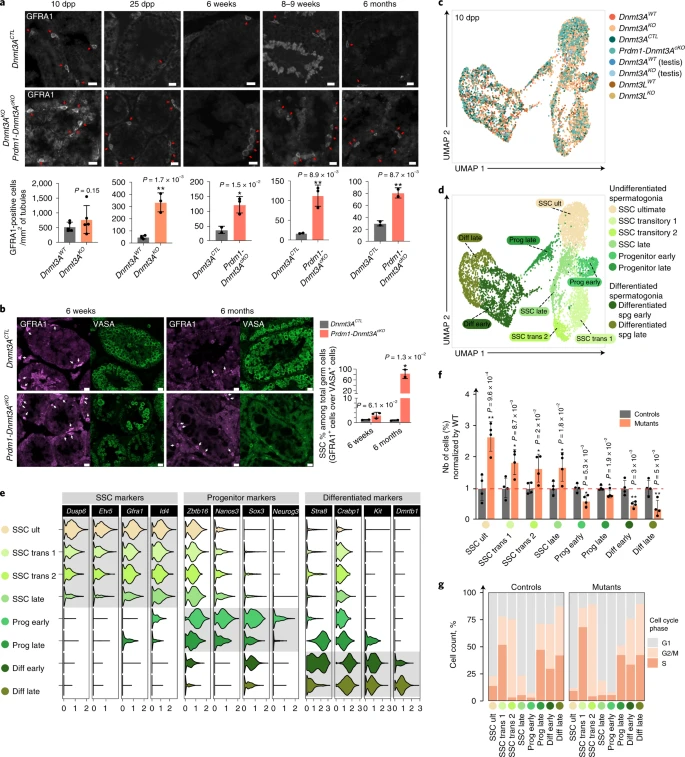
Figure 2: SSCs accumulate in absence of DNMT3A-dependent DNA methylation
a, Top: Representative image of GFRA1 (SSC marker) staining on testis sections from Dnmt3AKO, Prdm1-Dnmt3AcKO and controls at indicated ages. Scale bar, 20 μm. (Bottom) Quantification of GFRA1-positive cells per square millimeter of tubule. Dnmt3AWT: 10 dpp n = 5, 25 dpp n = 3. Control genotypes: 6 weeks n = 2 (Dnmt3AKO/WT; Prdm1-CreTg/0 and Dnmt3A2lox/KO; Prmd1-Cre0/0), 8–9 weeks n = 3 (n = 2 Dnmt3A2lox/KO; Prmd1-Cre0/0 and n = 1 Dnmt3A2lox/WT; Prdm1-Cre0/0), 6 months n = 2 (Dnmt3AKO/WT; Prmd1-CreTg/0 and Dnmt3A2lox/WT; Prmd1-Cre0/0). Dnmt3AKO: 10 dpp n = 5, 25 dpp n = 3. Prdm1-Dnmt3AcKO: 6 weeks n = 3, 8–9 weeks n = 3, 6 months n = 2. Data are mean ± s.d. (black bar). One-sided Student’s t test over WT: *P < 0.05, **P < 0.005. b, Left: Representative microscopy images of GFRA1 (purple) and VASA (green) immunodetection at 6 weeks and 6 months. Scale bars, 20 μm. (Right) Quantification of percentage of GFRA1-positive cells (SSCs) among VASA-positive cells (germ cells). 6 weeks: n = 2 control genotypes (Dnmt3AKO/WT; Prdm1-CreTg/0 and Dnmt3A2lox/KO; Prmd1-Cre0/0) and n = 3 Prdm1-Dnmt3AcKO. 6 months: n = 2 control genotypes (Dnmt3AKO/WT; Prmd1-CreTg/0 and Dnmt3A2lox/WT; Prmd1-Cre0/0) and n = 2 Prdm1-Dnmt3AcKO. Data are mean ± s.d. (black bar), individual points represent biological replicates (one-sided Student’s t-test over WT * P < 0.05). c, UMAP dimensionality reduction of scRNA-seq-integrated data from 10 dpp germ cells (n = 6,638). Colors represent eight genotypes and conditions. All conditions correspond to FACS-enriched germ cells, except for ‘testis’ (all testicular cells were analyzed). d, Unbiased cell clustering onto an UMAP representation of scRNA-seq data demonstrated eight germ cell clusters (from clusters 0 to 13 of unbiased cell clustering in Extended Data Fig. 5a). Diff, differentiated; spg, spermatogonia; ult, ultimate. e, Violin plots show mRNA level variation of different markers of SSCs, progenitors and differentiated spermatogonia among the eight germ cell clusters. f, Bar plot showing changes in germ cell cluster frequencies for pooled mutants normalized by controls. Data are mean ± s.d. (black bar) from four mutant samples (Dnmt3AKO, Prdm1-Dnmt3AcKO and Dnmt3LKO FACS-enriched germ cells and Dnmt3AKO all testicular cells) and four control samples (Dnmt3AWT, Dnmt3ACTL and Dnmt3LWT FACS-enriched germ cells and Dnmt3AWT all testicular cells). Statistical comparison was performed by one-sided Student’s t test over WT: NS, nonsignificant; * P < 0.05; ** P < 0.005; *** P < 0.005. g, Comparison of cell cycle signature for each germ cell cluster.
Licensed under: https://creativecommons.org/licenses/by/4.0/
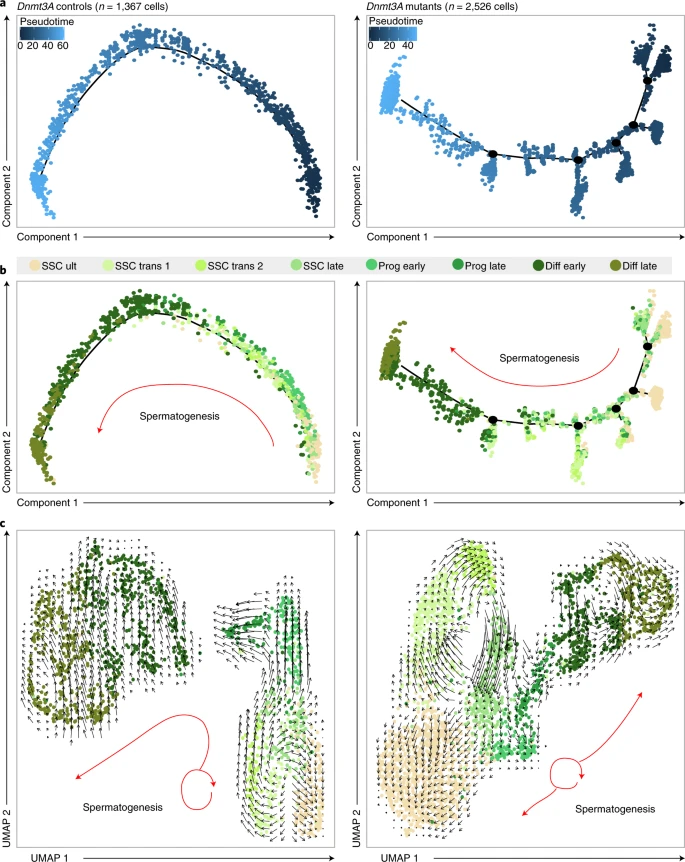
Figure 3: DNMT3A is essential to SSC plasticity
a,b, Pseudotime trajectory of germ cells FACS-sorted from 10 dpp testes. Left: Dnmt3A control germ cells (n = 874 Dnmt3AWT and n = 493 Dnmt3ACTL). Right: Dnmt3A mutant germ cells (n = 1,514 Dnmt3AKO and n = 1,012 Prdm1-Dnmt3AcKO). Cells are ordered from beginning (dark blue) to end (light blue) (a) or colored following cluster allocation (see key above) (b). c, Visualization of RNA velocity analysis on UMAP plot of control germ cells (left) and Dnmt3A mutant germ cells (right). Direction and size of arrows predict the future cellular state, based on transcriptional trajectories.
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 3: DNA methylation regulates SSC plasticity by limiting enhancer activity
Transcriptome: Bulk RNA-Sequencing
Species
| Species |
|---|
| Mouse |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | 10 days postpartum | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Mouse |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000016: male germ line stem cell | A stem cell that is the precursor of male gametes. | Mouse |
Images
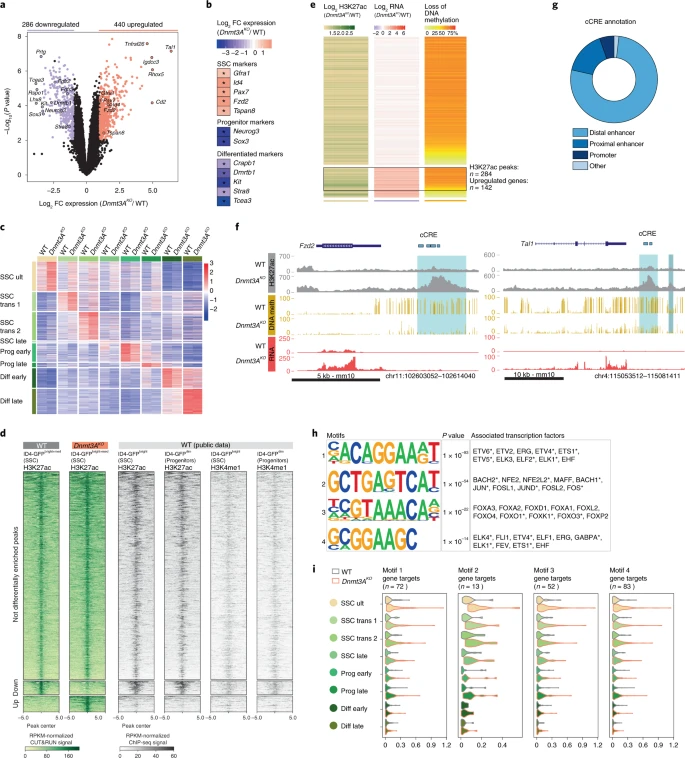
Figure 4: DNA methylation controls stem cell gene expression by limiting enhancer activity in SSCs
a, Volcano plots showing differential RNA levels between Dnmt3AKO and WT ID4-eGFPbright+med cells (10 dpp). Red: upregulated genes (440), purple: downregulated genes (286) (FDR < 5% and FC > 2, n = 3 biological replicates/genotype). b, RNA-seq heatmap of selected markers (FDR < 5% and FC > 2). c, scRNA-seq heatmap shows normalized expression of markers characterizing each germ cell population in corresponding Dnmt3AKO and WT cell types. d, (Left) Heatmap showing H3K27ac enrichment in reads per kilobase per million (RPKM)-normalized CUT&RUN signal from Dnmt3AKO and WT ID4-GFPbright+med germ cells (10 dpp) (±5 kb from peak center). Peak categories: not differentially enriched (Dnmt3AKO = WT), down-enriched (Dnmt3AKO < WT), up-enriched (Dnmt3AKO > WT) (FDR < 5% and FC > 1). Biological replicates: n = 3 Dnmt3AKO, n = 4 WT. (Right) H3K27ac and H3K4me1 enrichment from public ID4-eGFPbright and ID4-eGFPdim chromatin immunoprecipitation sequencing (ChIP-seq)36 (RPKM-normalized signal). e, Heatmap focusing on (left) Dnmt3AKO-gained H3K27ac peaks (log2 FC over WT); (center) differential expression (log2 FC) of genes associated with H3K27ac peaks (TSS ± 5 kb from peak) between Dnmt3AKO and WT—ID4-eGFPbright+med RNA-seq; (right) percentage of DNA methylation loss in Dnmt3AKO versus WT on H3K27ac peak location—E18.5 WGBS. Rows are ordered by gene expression changes: top, not differentially expressed; middle, upregulated; bottom, downregulated. f, Genomic annotations of ENCODE candidate cCREs overlapping with Dnmt3AKO-specific H3K27ac peaks associated with >30% DNA methylation (meth) loss and upregulated genes. g, Genome browser representations of H3K27ac enrichment (gray), DNA methylation (yellow) and RNA expression (red) for Dnmt3AKO and WT over the Fzd2 (left) and Tal1 (right) loci. Light-blue shade, Dnmt3AKO-specific H3K27ac peaks with >30% DNA methylation loss; gray-blue shade, de novo H3K27ac peaks at regions not controlled by DNA methylation. h, Left: Enriched motifs at Dnmt3AKO-specific H3K27ac peaks (n = 2,438). Middle: P values of motifs (binominal statistical test). Right: TFs with the best match for the motif. Asterisk indicates expression in SSCs from 10 dpp scRNA-seq data (Extended Data Fig. 8). i, Violin plots showing expression levels of TF gene targets in germ cell subtypes discriminated by scRNA-seq. TF gene targets were defined by association with Dnmt3AKO-specific H3K27ac peaks containing one or more motifs.
Licensed under: https://creativecommons.org/licenses/by/4.0/
