Origin and segregation of the human germline
Castillo-Venzor A, Penfold CA, Morgan MD, Tang WW, Kobayashi T, Wong FC, Bergmann S, Slatery E, Boroviak TE, Marioni JC, Surani MA., 22.05.2023
Abstract
Human germline-soma segregation occurs during weeks 2-3 in gastrulating embryos. Although direct studies are hindered, here, we investigate the dynamics of human primordial germ cell (PGCs) specification using in vitro models with temporally resolved single-cell transcriptomics and in-depth characterisation using in vivo datasets from human and nonhuman primates, including a 3D marmoset reference atlas. We elucidate the molecular signature for the transient gain of competence for germ cell fate during peri-implantation epiblast development. Furthermore, we show that both the PGCs and amnion arise from transcriptionally similar TFAP2A-positive progenitors at the posterior end of the embryo. Notably, genetic loss of function experiments shows that TFAP2A is crucial for initiating the PGC fate without detectably affecting the amnion and is subsequently replaced by TFAP2C as an essential component of the genetic network for PGC fate. Accordingly, amniotic cells continue to emerge from the progenitors in the posterior epiblast, but importantly, this is also a source of nascent PGCs.
Castillo-Venzor A, Penfold CA, Morgan MD, Tang WW, Kobayashi T, Wong FC, Bergmann S, Slatery E, Boroviak TE, Marioni JC, Surani MA. Origin and segregation of the human germline. Life Sci Alliance. 2023 May 22;6(8):e202201706. doi: 10.26508/lsa.202201706. PMID: 37217306; PMCID: PMC10203729.
Publication: https://doi.org/10.26508/lsa.202201706
 Disclaimer
Disclaimer
The publication Origin and segregation of the human germline by Castillo-Venzor A, Penfold CA, Morgan MD, Tang WW, Kobayashi T, Wong FC, Bergmann S, Slatery E, Boroviak TE, Marioni JC, Surani MA. is published under an open access license: https://creativecommons.org/licenses/by/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Transcriptional characterisation of PGC specification in embryoid bodies
Transcriptome: Single-cell RNA-Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| UBERON_0014374: embryoid body | Embryoid bodies (EBs) are three-dimensional aggregates of pluripotent stem cells. | Human |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000670: primordial germ cell | A primordial germ cell is a diploid germ cell precursors that transiently exist in the embryo before they enter into close association with the somatic cells of the gonad and become irreversibly committed as germ cells. | Human | |||
| CL_0002248: pluripotent stem cell | A pluripotent stem cell has the ability to form cells from all three germ layers (ectoderm, mesoderm, and endoderm). However, unlike totipotent stem cells, they cell can not generate all the cells of the whole organism such as placenta. | Human |
Images
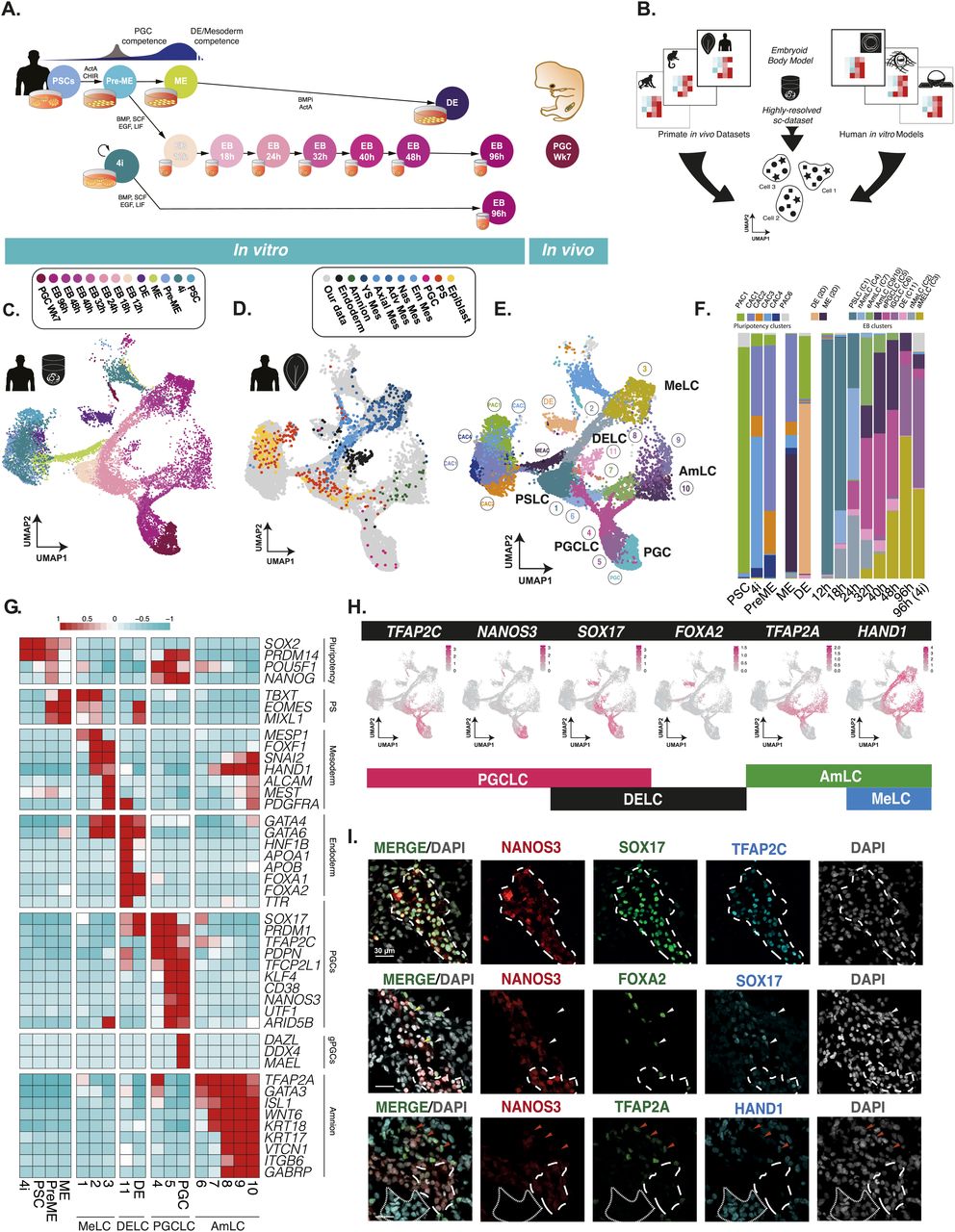
Figure 1: A highly resolved roadmap of PGC development and gastrulation
(A) Experimental design for highly resolved RNA sequencing (10X) of our established PGCLC model, alongside PGCLC-competent populations, and in vivo and in vitro reference cell types. PSC, pluripotent stem cells; PreME, pre-mesendoderm (transient PGCLC-competent cells); ME, mesendoderm; 4i, four-inhibitor, self-renewing PGCLC-competent cells; DE, definitive endoderm; PGC, week 7 human gonadal PGCs; EB, embryoid body. (B) A schematic for the integration of our data alongside other human in vitro models and primate gastrulation datasets used to generate a roadmap of PGC and early human development. (C) Integrated data representation of our samples as a UMAP projection highlighted by collection time and sample type. (D) Integrated representation of the aligned human CS7 gastrula data, highlighted by cell type plotted atop our data (in grey). (E) Louvain clustering of the integrated dataset identified 19 clusters and highlighted four key terminal lineages. (F) Quantification of the composition of transient and terminal lineages associated with individual samples. (G) Heatmaps of pseudo-bulk expression for key markers show that the embryoid body diversifies into mesoderm-like cells (MeLCs), definitive endoderm-like cells (DELCs), primordial germ cell-like cells (PGCLC), and amnion-like cells (AmLCs). (H) A minimal combination of key expression markers can be used to identify cell fates, with TFAP2C+/NANOS3+/SOX17+ representing PGCLCs; SOX17+/FOXA2+ endoderm fate, TFAP2A−/HAND1+ mesoderm, and TFAP2A+/HAND1− amnion fates. (I) Immunofluorescence of d4 EBs confirms expression patterns at the protein level, and the identity of PGCLCs, MeLCs, AmLCs, and DELCs.
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 2: Detection of PGC-competent population
Transcriptome: Single-cell RNA-Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| UBERON_0014374: embryoid body | Embryoid bodies (EBs) are three-dimensional aggregates of pluripotent stem cells. | Human |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0002248: pluripotent stem cell | A pluripotent stem cell has the ability to form cells from all three germ layers (ectoderm, mesoderm, and endoderm). However, unlike totipotent stem cells, they cell can not generate all the cells of the whole organism such as placenta. | Human | |||
| CL_0000670: primordial germ cell | A primordial germ cell is a diploid germ cell precursors that transiently exist in the embryo before they enter into close association with the somatic cells of the gonad and become irreversibly committed as germ cells. | Human |
Images
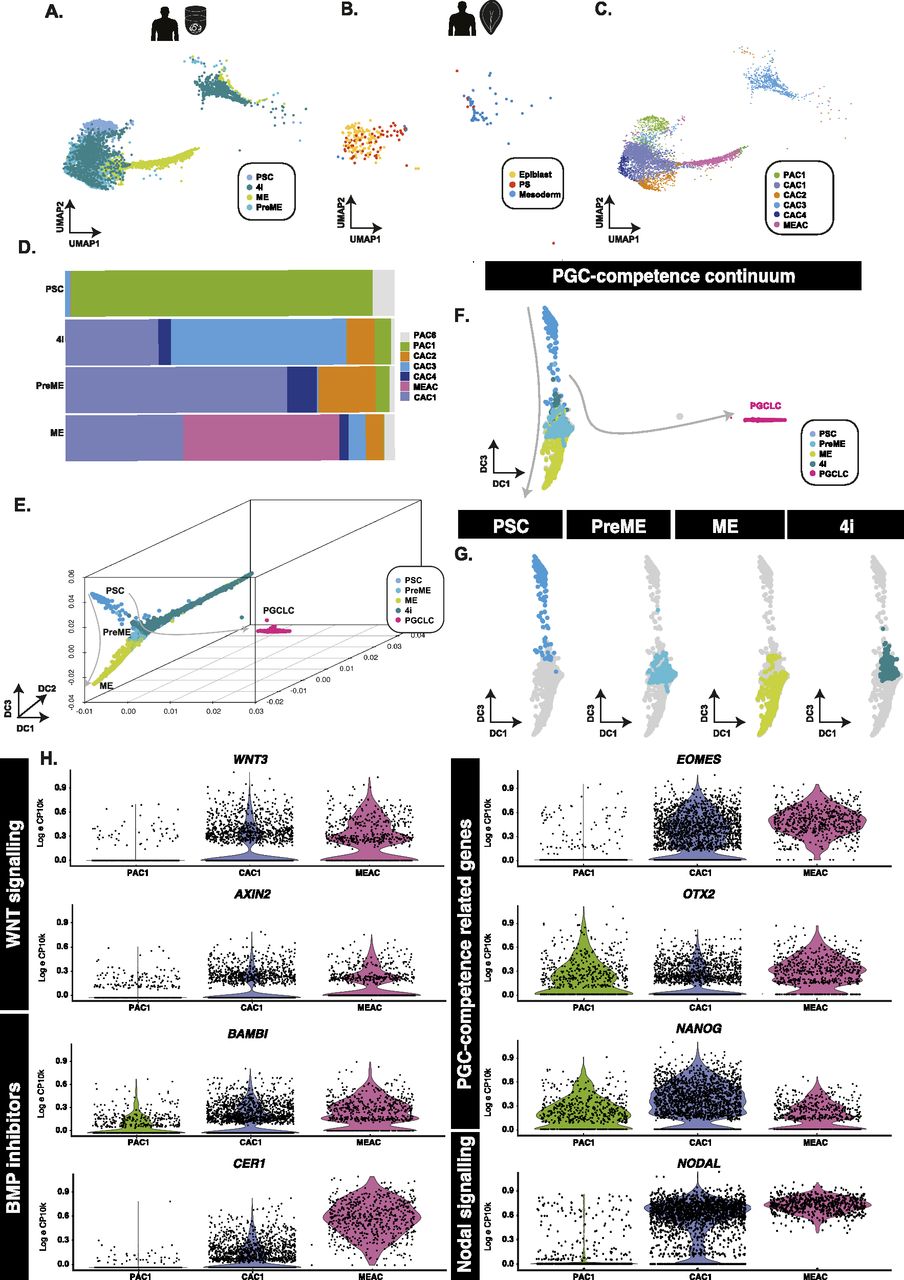
Figure 2: PGCLC-competent populations form a continuum of states
(A, B) Aligned UMAP representations of pluripotent and PGCLC-competent populations, alongside (B) human in vivo samples show that PSCs align best to pluripotent epiblast cells, whereas competent (PreME and 4i) align to both epiblast-like and primitive streak-like populations. (C, D) Clustering of competent and non-competent cells and quantification (D) identified six main populations that identify a PSC-associate cluster (PAC1) predominantly associated with the PSC samples, a mesendoderm-associated cluster primarily found within ME samples, and several overlapping putative competence-associated clusters (CAC1, 2, 3, and 4) found mainly in either 4i or PreME samples. (E, F, G) In 3D (E) and 2D (F) diffusion map representations, samples sit along a continuum of overlapping states with competent populations predominantly in the middle along DC3 (G). (H) Violin plots of putative competence genes related to WNT and BMP signalling reveal a heterogeneous signalling response.
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 3: Specification of PGCLCs in EBs represents a primitive streak-like stage
Transcriptome: Spatial Transcriptomics
Species
| Species |
|---|
| Marmoset |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0000379: embryo | An animal in the early stages of growth and differentiation that are characterized by cleavage, the laying down of fundamental tissues, and the formation of primitive organs and organ systems; especially: the developing human individual from the time of implantation to the end of the eighth week after conception. |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000670: primordial germ cell | A primordial germ cell is a diploid germ cell precursors that transiently exist in the embryo before they enter into close association with the somatic cells of the gonad and become irreversibly committed as germ cells. |
Images
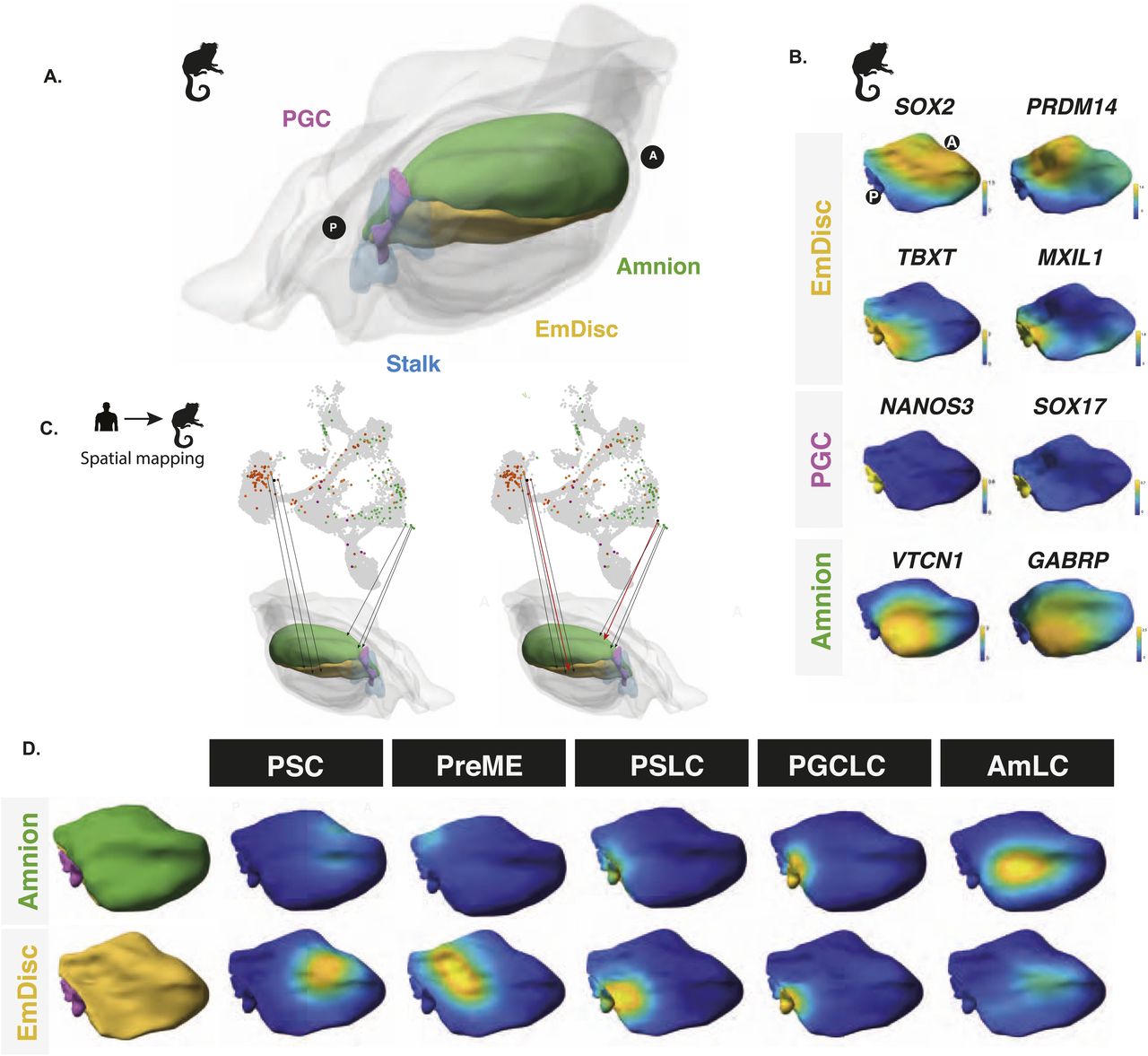
Figure 3: Spatial mapping of embryoid bodies to gastrulating marmoset embryos reveals a posterior bias
(A) Spatially resolved marmoset embryos at CS6 with the embryonic disc in yellow, amnion in green, PGCs in pink, and stalk in blue. Extraembryonic tissues are depicted in grey. (B) Expression analysis in the marmoset reference embryo at Carnegie Stage 6 shows that the anterior embryonic disc is SOX2 positive and posterior regions are TBXT positive. Specified PGCs show similar expression patterns to humans, with SOX17/NANOS3 expression, and amnion showing partial GABRP/VTCN1 expression. (C) After the alignment of datasets visualised here as a UMAP, mapping of human in vitro cells to the marmoset reference embryo was achieved using KNN-based methods in PC space, with PSCs mapping best to the anterior embryonic disc. (D) Competent populations show a distinct posterior bias, with PGCLCs showing strong localisation to the posterior-most marmoset PGC region and AmLCs mapping to the amnion.
Licensed under: https://creativecommons.org/licenses/by/4.0/
Data set 4: TFAP2A is a regulator of PGCLC fate
Transcriptome: Single-cell RNA-Sequencing
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| UBERON_0014374: embryoid body | Embryoid bodies (EBs) are three-dimensional aggregates of pluripotent stem cells. | Human |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0002248: pluripotent stem cell | A pluripotent stem cell has the ability to form cells from all three germ layers (ectoderm, mesoderm, and endoderm). However, unlike totipotent stem cells, they cell can not generate all the cells of the whole organism such as placenta. | Human |
Images
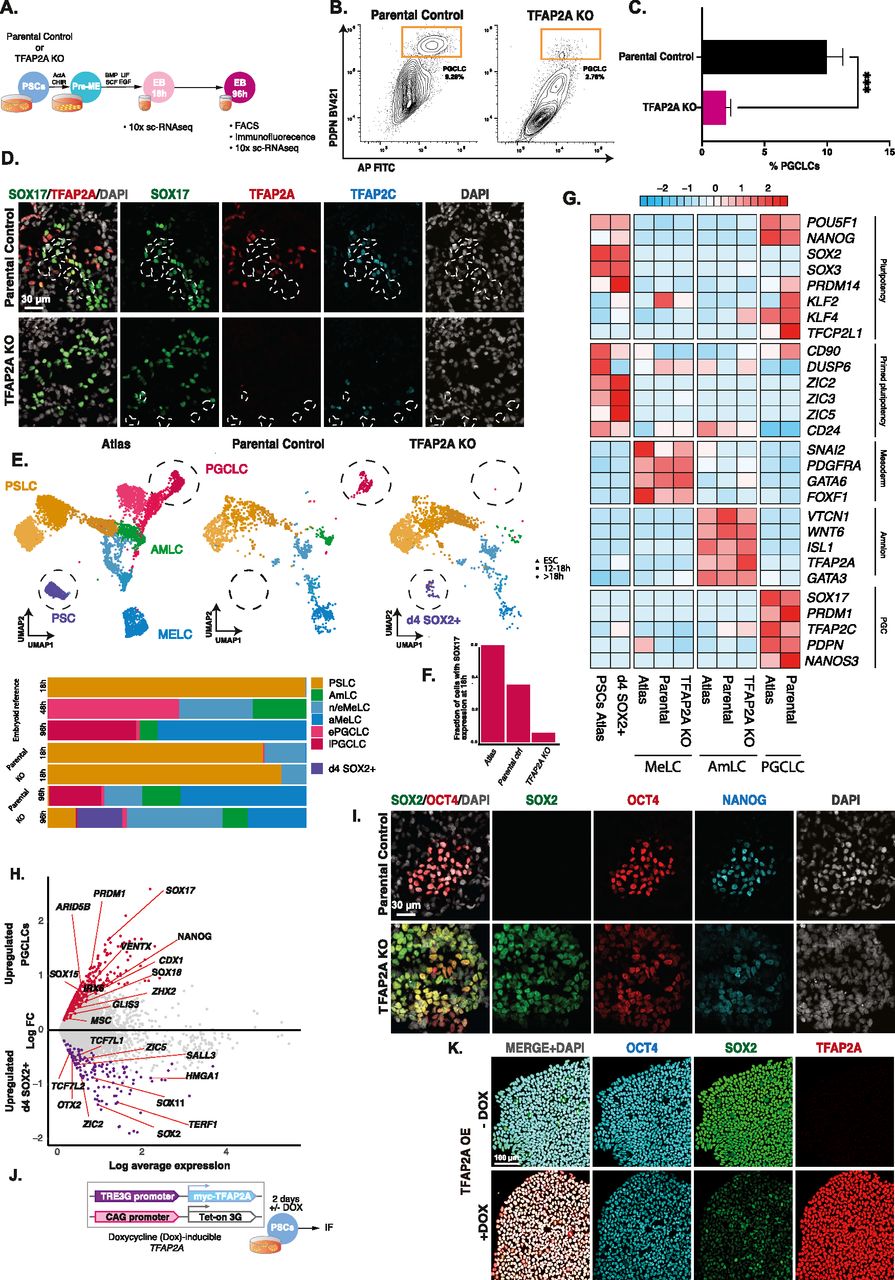
Figure 4: TFAP2A is a regulator of PGCLC fate
(A) Experimental design for testing the role of TFAP2A in PGC specification using TFAP2A knockout line. (B, C) FACS plots and quantification based on IF-labelled PDPN expression reveal a decrease in the % of PGCLCs induced in TFAP2A KO EBs compared with WT parental control. (D) Immunofluorescence shows co-expression for SOX17, TFAP2A, and TFAP2C in d4 EB. (E) Aligned UMAPs and bar plot quantification for the reference atlas versus H9 parental control and H9 TFAP2A KO further corroborate the drastic reduction in the numbers of PGCLCs in the knockout line. These results further suggest the emergence of a new SOX2+ population that occurs after 18 h and aligns with pluripotent stem cells in the reference atlas (D4 SOX2+). (F) Fraction of cells positive for SOX17 expression at the 18 h time point in reference atlas, parental control, and TFAP2A KO cells. (G) Row-normalised gene expression demonstrates consistent expression in AmLC and MeLC in the TFAP2A KO line. D4 SOX2+ cells show the expression of pluripotency genes. (H) Volcano plot for differentially expressed genes between the d4 SOX2+ cluster in TFAP2A KO versus PGCLCs in parental control. (I) Immunofluorescence of d4 parental EBs shows OCT4 NANOG double-positive cells (PGCLCs) but not in TFAP2A KO EBs; instead, there are OCT4, NANOG, and SOX2 triple-positive cells. (J) An inducible system to test the role of TFAP2A overexpression on SOX2 expression in PSCs. (K) Immunofluorescence for OCT4, SOX2, TFAP2A in PSCs after TFAP2A induction.
Licensed under: https://creativecommons.org/licenses/by/4.0/
