Genome-Wide Association Screening Determines Peripheral Players in Male Fertility Maintenance
Greither T, Behre HM, Herlyn H, 28.12.2022
Abstract
Deciphering the functional relationships of genes resulting from genome-wide screens for polymorphisms that are associated with phenotypic variations can be challenging. However, given the common association with certain phenotypes, a functional link should exist. We have tested this prediction in newly sequenced exomes of altogether 100 men representing different states of fertility. Fertile subjects presented with normal semen parameters and had naturally fathered offspring. In contrast, infertile probands were involuntarily childless and had reduced sperm quantity and quality. Genome-wide association study (GWAS) linked twelve non-synonymous single-nucleotide polymorphisms (SNPs) to fertility variation between both cohorts. The SNPs localized to nine genes for which previous evidence is in line with a role in male fertility maintenance: ANAPC1, CES1, FAM131C, HLA-DRB1, KMT2C, NOMO1, SAA1, SRGAP2, and SUSD2. Most of the SNPs residing in these genes imply amino acid exchanges that should only moderately affect protein functionality. In addition, proteins encoded by genes from present GWAS occupied peripheral positions in a protein-protein interaction network, the backbone of which consisted of genes listed in the Online Mendelian Inheritance in Man (OMIM) database for their implication in male infertility. Suggestive of an indirect impact on male fertility, the genes focused were indeed linked to each other, albeit mediated by other interactants. Thus, the chances of identifying a central player in male infertility by GWAS could be limited in general. Furthermore, the SNPs determined and the genes containing these might prove to have potential as biomarkers in the diagnosis of male fertility.
Greither T, Behre HM, Herlyn H. Genome-Wide Association Screening Determines Peripheral Players in Male Fertility Maintenance. Int J Mol Sci. 2022 Dec 28;24(1):524. doi: 10.3390/ijms24010524. PMID: 36613967; PMCID: PMC9820667.
Publication: https://doi.org/10.3390/ijms24010524
 Disclaimer
Disclaimer
The publication Genome-Wide Association Screening Determines Peripheral Players in Male Fertility Maintenance by Greither T, Behre HM, Herlyn H is published under an open access license: https://creativecommons.org/licenses/by-nc/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Whole exome sequencing identifying single-nucleotide polymorphisms (SNPs) in 100 infertile and normal-fertile men
Exome: Whole Exome Sequencing
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0000089: blood | adult, normal-fertile | 1: The fluid that circulates in the heart, arteries, capillaries, and veins of a vertebrate animal carrying nourishment and oxygen to and bringing away waste products from all parts of the body. 2: A comparable fluid of an invertebrate. | Human | 30 |
| BTO_0000089: blood | adult, infertile | 1: The fluid that circulates in the heart, arteries, capillaries, and veins of a vertebrate animal carrying nourishment and oxygen to and bringing away waste products from all parts of the body. 2: A comparable fluid of an invertebrate. | Human | 70 |
Images
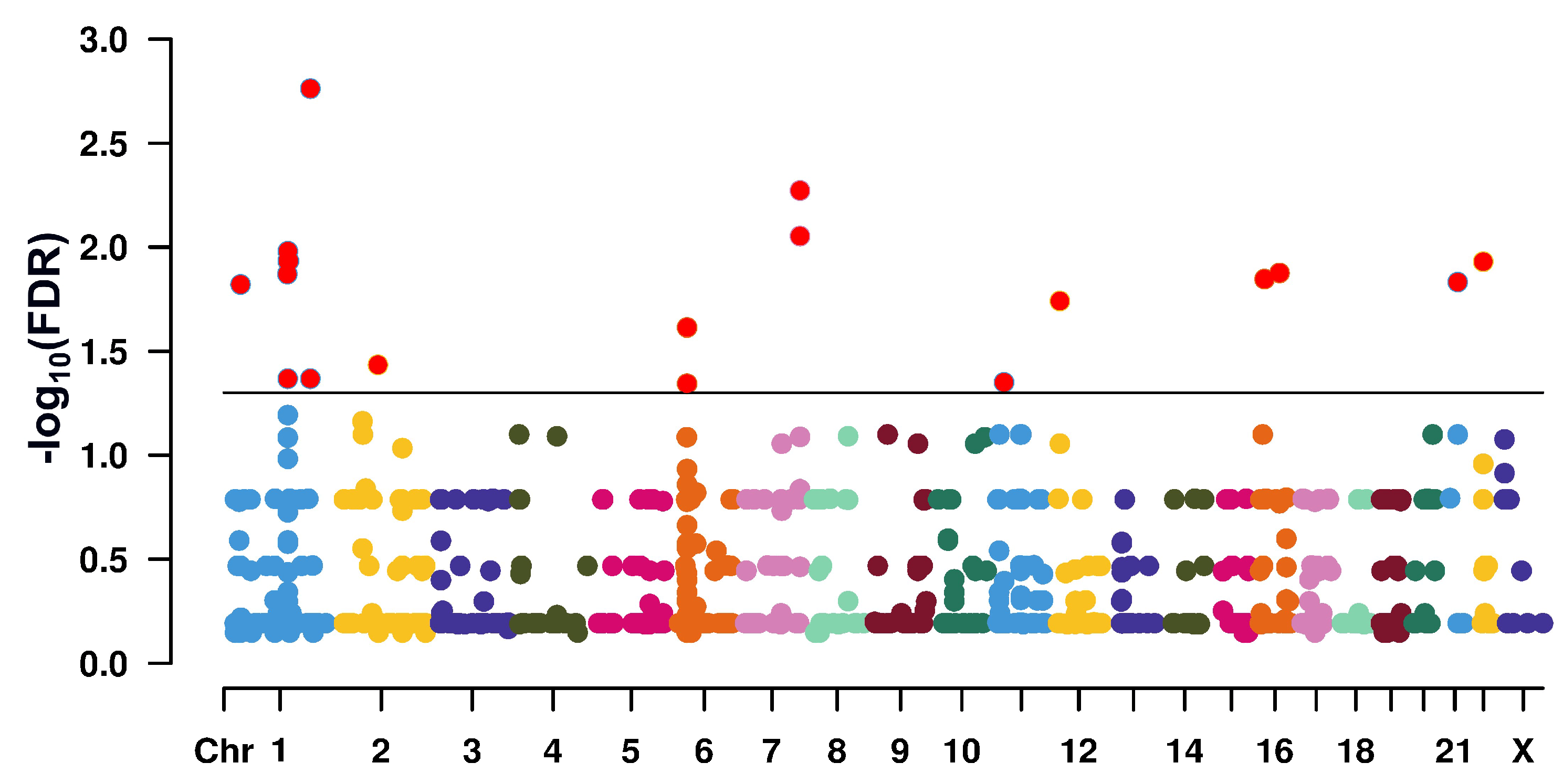
Figure 1: Manhattan plot giving the genomic position of single-nucleotide polymorphisms (SNPs) with differing allele frequencies between normal-fertile and infertile men
Only SNPs (N = 840) which had been genotyped in all 100 probands were considered. The Y-axis refers to —log10-transformed false discovery rates (FDRs). The horizontal line at 1.3 corresponds to the 5% significance threshold. SNPs exceeding the threshold are highlighted in red. Coloration of the remainder SNPs reflects their localization on different chromosomes. For clarity, chromosome (Chr) numbering is incomplete.
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
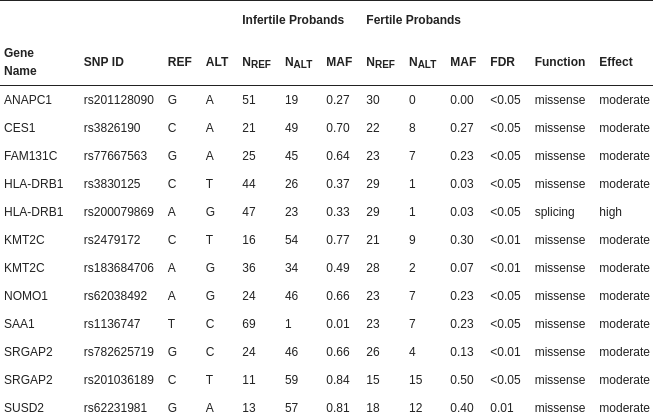
Table 1: Single-nucleotide polymorphisms with different allele frequencies in infertile and fertile men
Inference of false discovery rate (FDR) values followed the procedure by [43]. Abbreviations: ALT—alternative allele; MAF—minor allele frequency; NALT—number of observations of the alternative allele; NREF—number of observations of the reference allele; REF—reference allele; SNP ID—single-nucleotide polymorphism identifier as verified in the dbSNP database of the National Center for Biotechnology Information (NCBI).
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
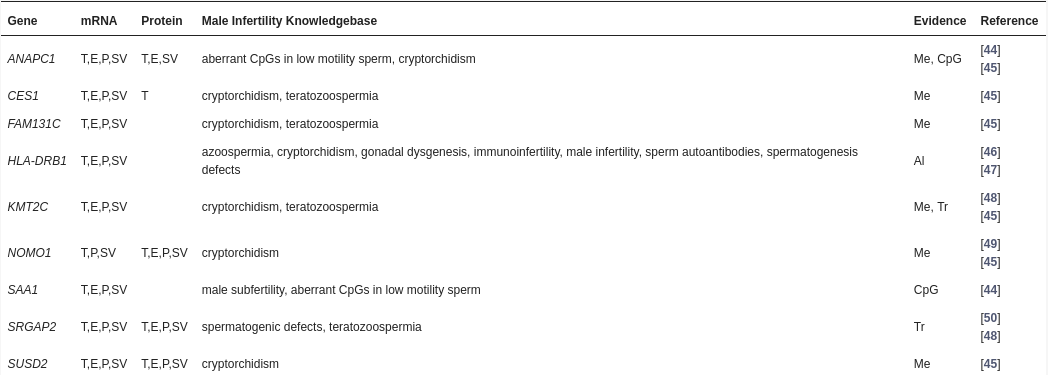
Table 2: Evidence suggesting fertility-relevance of genes harboring SNPs from present GWAS
Expression data (mRNA, protein) refers to The Human Protein Atlas. Abbreviations: Al—allele frequencies; CpG—CpG pattern; E—epididymis; Me—methylation; P—prostate; T—Testis; Tr—transcript abundance; SNP—single-nucleotide polymorphism; SV—seminal vesicle.
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
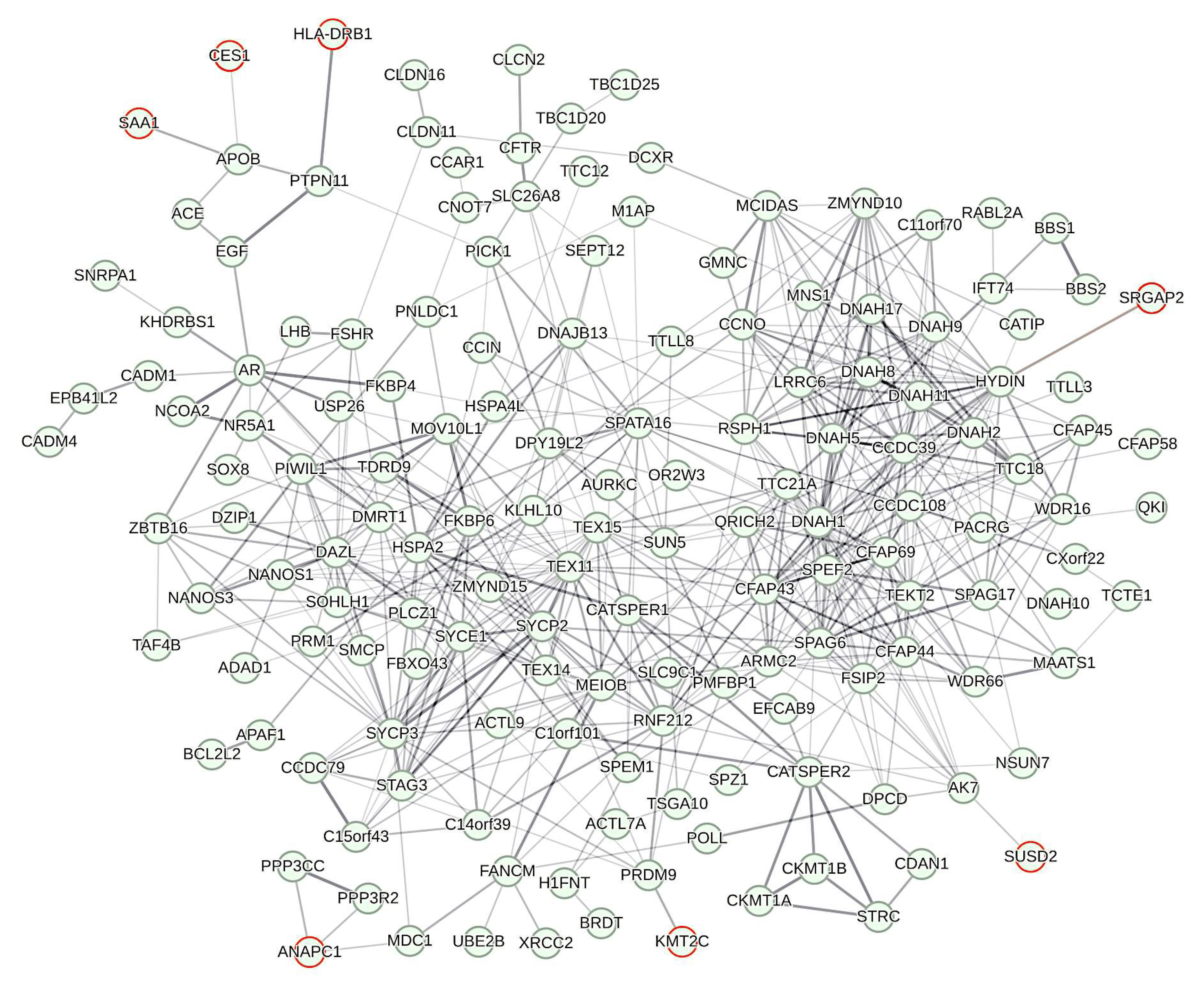
Figure 2: Largest connected component (LCC) of the protein–protein interaction (PPI) network reconstructed from genes implicated in male infertility
Most of the proteins included refer to genes reported in NCBI OMIM for their involvement in male infertility. Proteins to seven out of nine genes emerging from present GWAS (red circles) are included in the LCC, thereby occupying peripheral positions. Correspondingly, the number of PPIs per each of these genes is 1.286, whereas average node degree is 3.628 across the entire LCC. The LCC has 166 nodes between which 566 PPIs extend. Compared to the expected number of edges (N = 79) this represents a highly significant enrichment (p < 1 × 10−16). Average clustering coefficient across the LCC is 0.518. Strength of edges gives increments of minimum confidence (0.150, 0.400, 0.700, 0.900). Network reconstruction was conducted with STRING v11.5 using standard settings.
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
