The human sperm proteome-Toward a panel for male fertility testing
Greither T, Dejung M, Behre HM, Butter F, Herlyn H, 13.04.2023
Abstract
Background: Although male factor accounts for 40%-50% of unintended childlessness, we are far from fully understanding the detailed causes. Usually, affected men cannot even be provided with a molecular diagnosis. Objectives: We aimed at a higher resolution of the human sperm proteome for better understanding of the molecular causes of male infertility. We were particularly interested in why reduced sperm count decreases fertility despite many normal-looking spermatozoa and which proteins might be involved. Material and methods: Applying mass spectrometry analysis, we qualitatively and quantitatively examined the proteomic profiles of spermatozoa from 76 men differing in fertility. Infertile men had abnormal semen parameters and were involuntarily childless. Fertile subjects exhibited normozoospermia and had fathered children without medical assistance. Results: We discovered proteins from about 7000 coding genes in the human sperm proteome. These were mainly known for involvements in cellular motility, response to stimuli, adhesion, and reproduction. Numbers of sperm proteins showing at least threefold deviating abundances increased from oligozoospermia (N = 153) and oligoasthenozoospermia (N = 154) to oligoasthenoteratozoospermia (N = 368). Deregulated sperm proteins primarily engaged in flagellar assembly and sperm motility, fertilization, and male gametogenesis. Most of these participated in a larger network of male infertility genes and proteins. Discussion: We expose 31 sperm proteins displaying deviant abundances under infertility, which already were known before to have fertility relevance, including ACTL9, CCIN, CFAP47, CFAP65, CFAP251 (WDR66), DNAH1, and SPEM1. We propose 18 additional sperm proteins with at least eightfold differential abundance for further testing of their diagnostic potential, such as C2orf16, CYLC1, SPATA31E1, SPATA31D1, SPATA48, EFHB (CFAP21), and FAM161A. Conclusion: Our results shed light on the molecular background of the dysfunctionality of the fewer spermatozoa produced in oligozoospermia and syndromes including it. The male infertility network presented may prove useful in further elucidating the molecular mechanism of male infertility.
Greither T, Dejung M, Behre HM, Butter F, Herlyn H. The human sperm proteome-Toward a panel for male fertility testing. Andrology. 2023 Oct;11(7):1418-1436. doi: 10.1111/andr.13431. Epub 2023 Apr 13. PMID: 36896575.
Publication: https://doi.org/10.1111/andr.13431
 Disclaimer
Disclaimer
The publication The human sperm proteome-Toward a panel for male fertility testing by Greither T, Dejung M, Behre HM, Butter F, Herlyn H is published under an open access license: https://creativecommons.org/licenses/by-nc/4.0/. Permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1: Examination of proteomic profiles of spermatozoa from infertile men using mass spectrometry analysis
Proteome: Mass Spectrometry Analysis
Species
| Species |
|---|
| Human |
Tissue Types
| BRENDA tissue ontology | Maturity | Description | Species | Replicates |
|---|---|---|---|---|
| BTO_0001363: testis | adult, normozoospermic | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 38 |
| BTO_0001363: testis | adult, oligozoospermic (O) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 21 |
| BTO_0001363: testis | adult, oligoasthenozoospermic (OA) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 7 |
| BTO_0001363: testis | adult, oligoasthenoteratozoospermic (OAT) | A typically paired male reproductive gland that produces sperm and that in most mammals is contained within the scrotum at sexual maturity. | Human | 10 |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000019: sperm | A mature male germ cell that develops from a spermatid. | Human |
Images
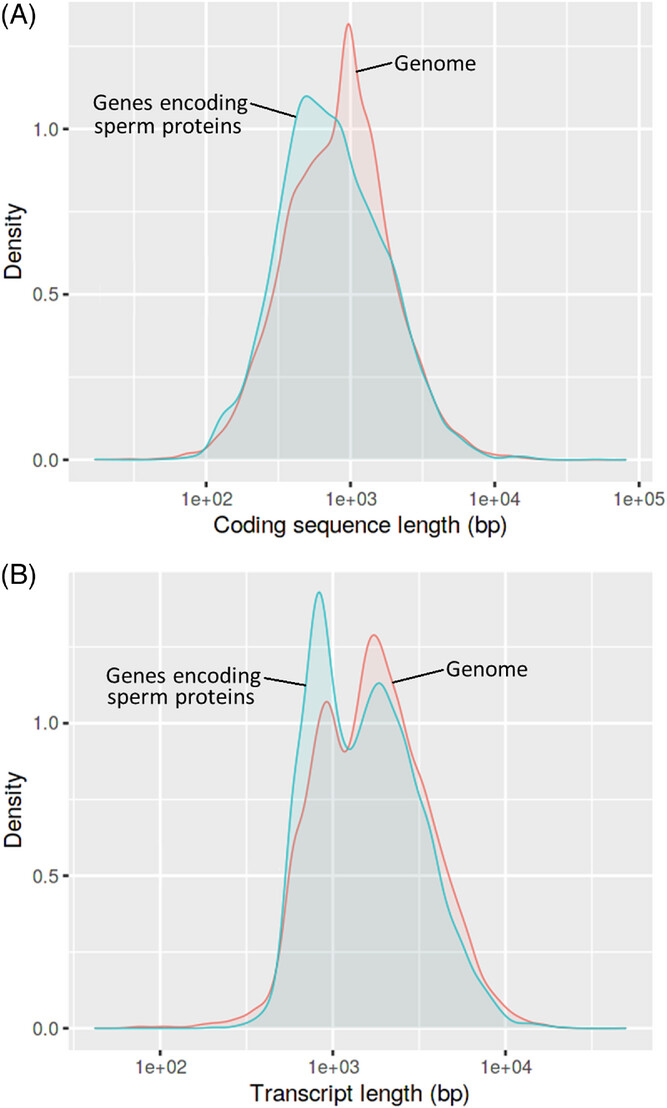
Figure 1: Distributions of coding sequence length (A) and transcript length (B) in human genes encoding sperm proteins, relative to the genome
Shifts to the left indicate overall lower values in 6939 sperm protein-coding genes mapped by ShinyGO v0.76 (Table S2). Chi-square tests contrast patterns in sperm protein-coding genes and all other genes were highly significant (false discovery rate [FDR] <0.001, each).
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
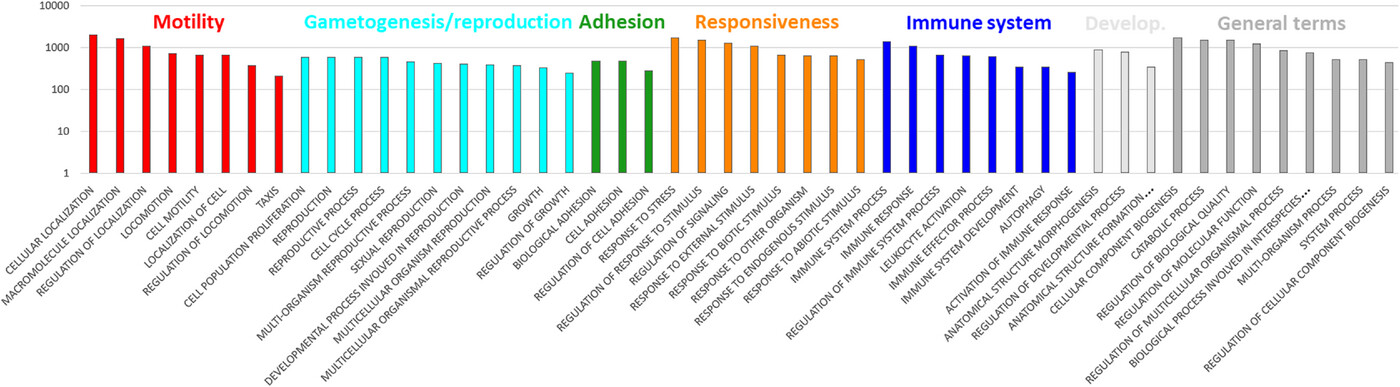
Figure 2: Representation of higher level biological process gene ontologies (GOs) in the human sperm proteome
Shown are 50 GOs with highest frequencies of occurrence. Included are 6939 sperm protein-coding genes mapped by ShinyGO v0.76. See Table S2 for the full terms of two GOs abbreviated in the above chart. The same table includes the remaining 50 GOs, which occur with lower frequency, and the genes subsumed under each GO.
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
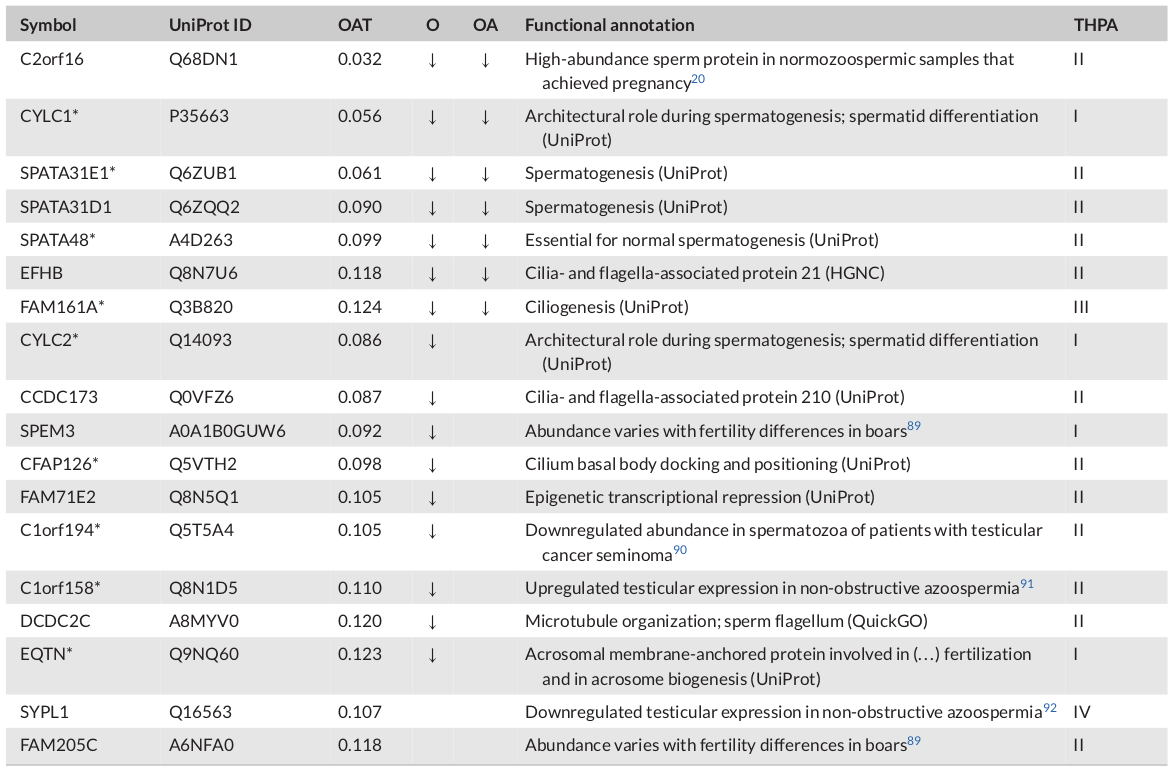
Table 1: Novel candidate markers: sperm proteins with the greatest decline in abundance
Note: Listed are sperm proteins without previous reference to male fertility impairment (NCBI Online Mendelian Inheritance in Men [OMIM]) that showed at least eightfold decreased abundances in oligoasthenoteratozoospermia (OAT) (false discovery rate [FDR] ≤ 0.05, t-test). Arrows indicate the direction abundance changes in spermatozoa of infertile men diagnosed with oligozoospermia (O) and oligoasthenozoospermia (OA). Changes refer to protein abundances in normozoospermic sperm proteins of fertile men. Asterisks highlight proteins included in the largest connected component shown in Figure 6. Categories on the left refer to transcript abundances reported in The Human Protein Atlas v22 (THPA): male germline-specific (I), male germline-biased (II), elevated in male germline and somatic cell types, which unlikely (III) or possibly were contained in the samples (IV).
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
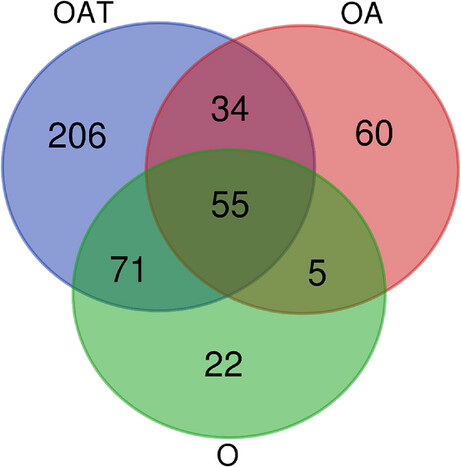
Figure 3: Numbers of shared and unique protein groups showing differential abundances in mass spectrometry (MS) analyses of oligozoospermic (O), oligoasthenozoospermic (OA), and oligoasthenoteratozoospermic spermatozoa (OAT)
For consideration, a protein group had to show at least threefold differential abundance under infertility compared to levels in normozoospermic spermatozoa (false discovery rate [FDR] ≤ 0.05, t-test). The number of unique protein groups increased with growing complexity of the infertility diagnosis, and thus from O via OA to OAT. The Venn diagram was drawn using https://bioinformatics.psb.ugent.be/webtools/Venn/.
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
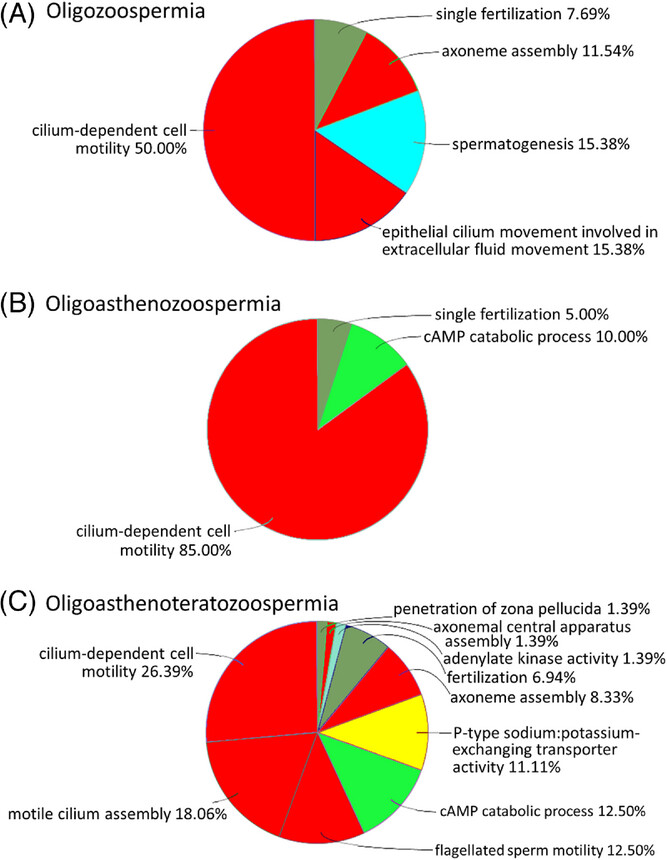
Figure 4: Representation of biological process gene ontologies (GOs) in sperm proteins exhibiting deviant abundances in oligozoospermia (A), oligoasthenozoospermia (B), and oligoasthenoteratozoospermia (C)
Only highly significant results from overrepresentation test were considered (false discovery rate [FDR] ≤ 0.001). Highest diversity of overrepresented GOs was found in the most complex diagnosis, oligoasthenoteratozoospermia. GOs relating to sperm flagellum and motility made up more than two-thirds of the annotations in all diagnoses (red fill). Additional GOs overrepresented were relatable to male gametogenesis (turquoise), cAMP metabolism (light green), fertilization (olive green), and ion exchange (yellow). The lists tested were compiled from protein groups showing at least threefold differential abundances in spermatozoa of infertile men with respective diagnoses, relative to normozoospermic spermatozoa of fertile men (FDR ≤ 0.05, t-test). Analyses used ClueGO v2.5.9 within Cytoscape v3.9.1.
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
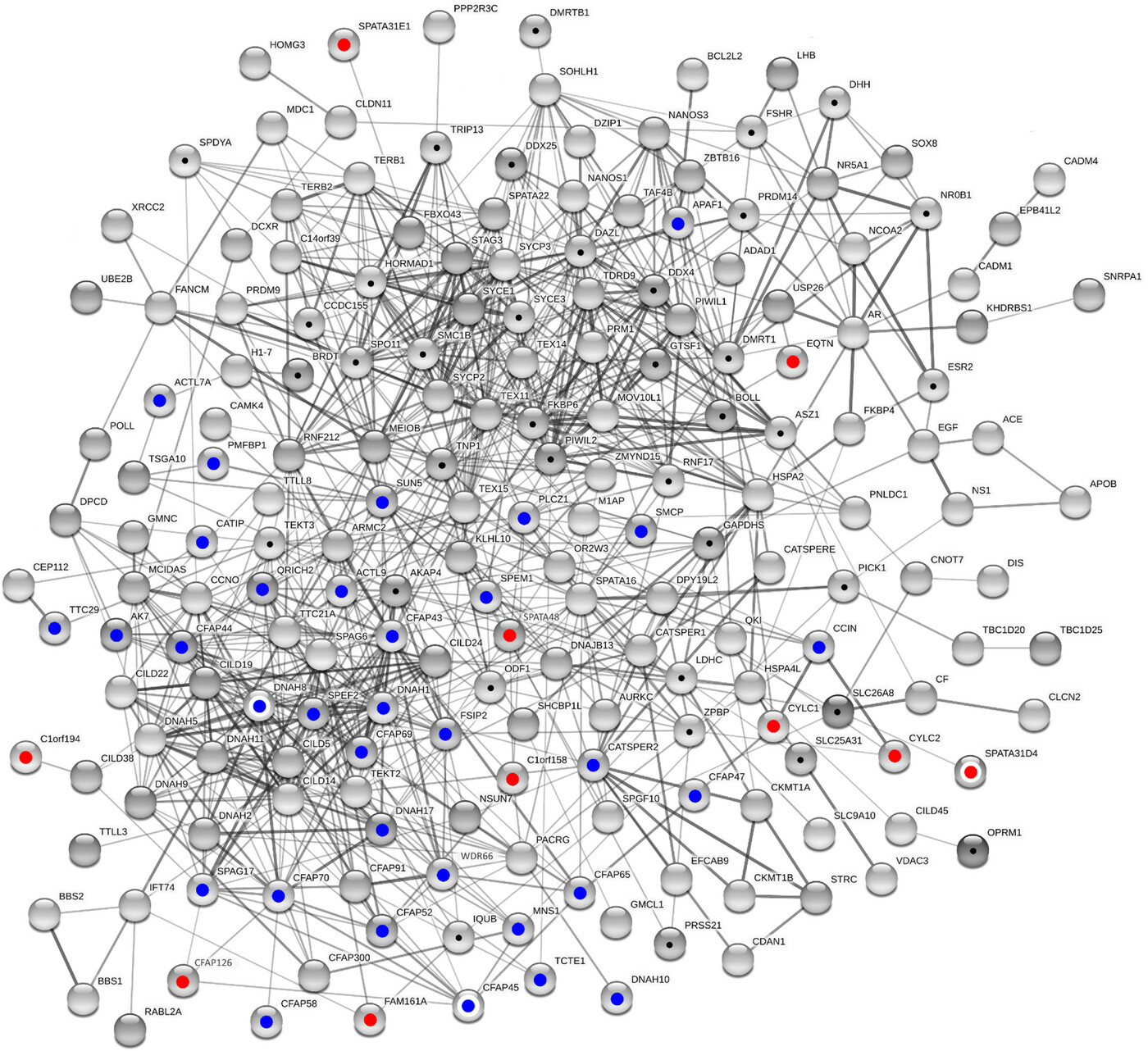
Figure 5: Male infertility network
Shown is the largest connected component from network reconstruction using standard settings in STRING v11.5. Balls give proteins and lines interactions. Thickness of lines corresponds to confidence levels. The reconstruction is based on a list merged from validated (blue dots) and novel candidate markers (red dots) according to the present study, proteins with elevated fertility relevance probability (black dots) according to previous calculations,19 and further proteins with prior mention in the male fertility context (Online Mendelian Inheritance in Men [OMIM]). White circles surrounding colored filling highlight members of protein groups as specified in Table 5. In its current state, the male infertility network contains 192 proteins connected by 891 edges.
Licensed under: https://creativecommons.org/licenses/by-nc/4.0/
