Recessive HYDIN Mutations Cause Primary Ciliary Dyskinesia without Randomization of Left-Right Body Asymmetry
Heike Olbrich, Miriam Schmidts, Claudius Werner, Alexandros Onoufriadis, Niki T.Loges, Johanna Raidt, Nora Fanni Banki, Amelia Shoemark, Tom Burgoyne, Saeed Al Turki, Matthew E.Hurles, UK10K Consortium, Gabriele Köhler, Josef Schroeder, Gudrun Nürnberg, Peter Nürnberg, Eddie M.K.Chung, Richard Reinhardt…Heymut Omran, 27.09.2012
Abstract
Primary ciliary dyskinesia (PCD) is a genetically heterogeneous recessive disorder characterized by defective cilia and flagella motility. Chronic destructive-airway disease is caused by abnormal respiratory-tract mucociliary clearance. Abnormal propulsion of sperm flagella contributes to male infertility. Genetic defects in most individuals affected by PCD cause randomization of left-right body asymmetry; approximately half show situs inversus or situs ambiguous. Almost 70 years after the hy3 mouse possessing Hydin mutations was described as a recessive hydrocephalus model, we report HYDIN mutations in PCD-affected persons without hydrocephalus. By homozygosity mapping, we identified a PCD-associated locus, chromosomal region 16q21-q23, which contains HYDIN. However, a nearly identical 360 kb paralogous segment (HYDIN2) in chromosomal region 1q21.1 complicated mutational analysis. In three affected German siblings linked to HYDIN, we identified homozygous c.3985G>T mutations that affect an evolutionary conserved splice acceptor site and that subsequently cause aberrantly spliced transcripts predicting premature protein termination in respiratory cells. Parallel whole-exome sequencing identified a homozygous nonsense HYDIN mutation, c.922A>T (p.Lys307∗), in six individuals from three Faroe Island PCD-affected families that all carried an 8.8 Mb shared haplotype across HYDIN, indicating an ancestral founder mutation in this isolated population. We demonstrate by electron microscopy tomography that, consistent with the effects of loss-of-function mutations, HYDIN mutant respiratory cilia lack the C2b projection of the central pair (CP) apparatus; similar findings were reported in Hydin-deficient Chlamydomonas and mice. High-speed videomicroscopy demonstrated markedly reduced beating amplitudes of respiratory cilia and stiff sperm flagella. Like the hy3 mouse model, all nine PCD-affected persons had normal body composition because nodal cilia function is apparently not dependent on the function of the CP apparatus.
OLBRICH, Heike, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. The American Journal of Human Genetics, 2012, 91. Jg., Nr. 4, S. 672-684.
Publication: https://doi.org/10.1016/j.ajhg.2012.08.016
 Disclaimer
Disclaimer
The publication Recessive HYDIN Mutations Cause Primary Ciliary Dyskinesia without Randomization of Left-Right Body Asymmetry by Heike Olbrich, Miriam Schmidts, Claudius Werner, Alexandros Onoufriadis, Niki T.Loges, Johanna Raidt, Nora Fanni Banki, Amelia Shoemark, Tom Burgoyne, Saeed Al Turki, Matthew E.Hurles, UK10K Consortium, Gabriele Köhler, Josef Schroeder, Gudrun Nürnberg, Peter Nürnberg, Eddie M.K.Chung, Richard Reinhardt…Heymut Omran is published under an open access license: https://creativecommons.org/licenses/by/3.0/. Granted rights: share — copy and redistribute the material in any medium or format and adapt — remix, transform, and build upon the material for any purpose, even commercially.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1:
Other: Functional Study
Species
| Species |
|---|
| Human |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:0012265: Ciliary dyskinesia |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000066: epithelial cell | Adult | Respiratory epithelial cells and nasal epithelial cells | Human | ||
| CL_0000019: sperm | Adult | Human |
Images
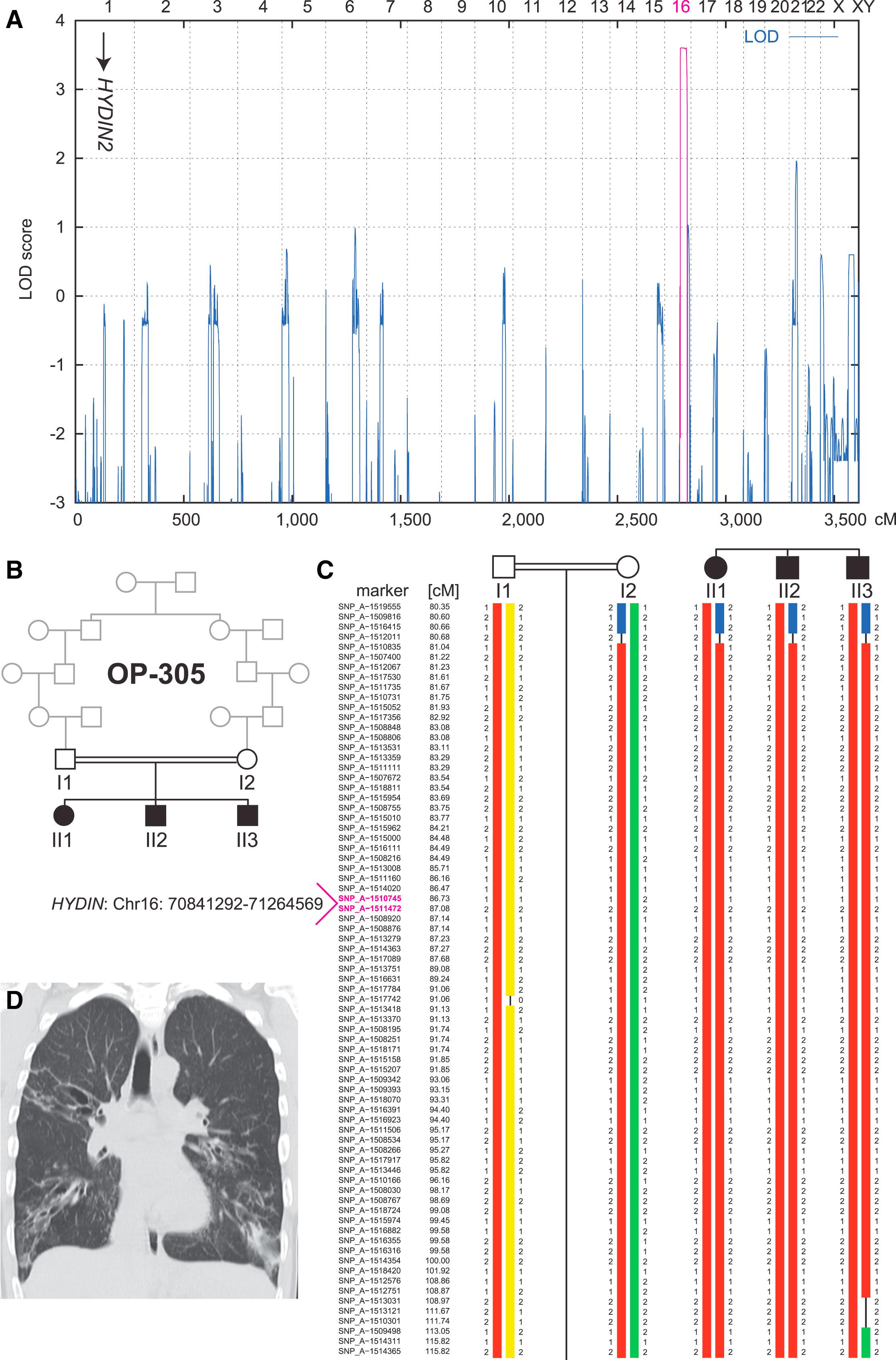
Figure 1: Linkage and Haplotype Analysis of Family OP-305
(A) Genome-wide linkage analysis of the consanguineous family OP-305 identifies a PCD gene locus on chromosome 16, which contains HYDIN. The position of the pseudogene HYDIN2 on chromosome 1 is marked with an arrow. A schematic representation of genome-wide LOD scores is shown. LOD scores are given along the y axis relative to genomic position in cM on the x axis. Note the significant peak (LOD = 3.6) in the region on chromosome 16.</br>(B) Pedigree of the OP-305 family.</br>(C) Haplotype reconstruction for the linkage region on chromosome 16. Disease-associated haplotypes are in red. The flanking markers SNP_A-1516415 (rs2221744) and SNP_A-1509498 (rs433325) span a region of 32.4 cM (corresponding to a genomic region of 21.8 Mb).</br>(D) High-resolution computed-tomography scans of individual OP-305 II1 show bronchiectasis of the right upper lobe, middle lobe, and lower lobes, peribronchial thickening, and airway collapse.
Licensed under: https://creativecommons.org/licenses/by/3.0/
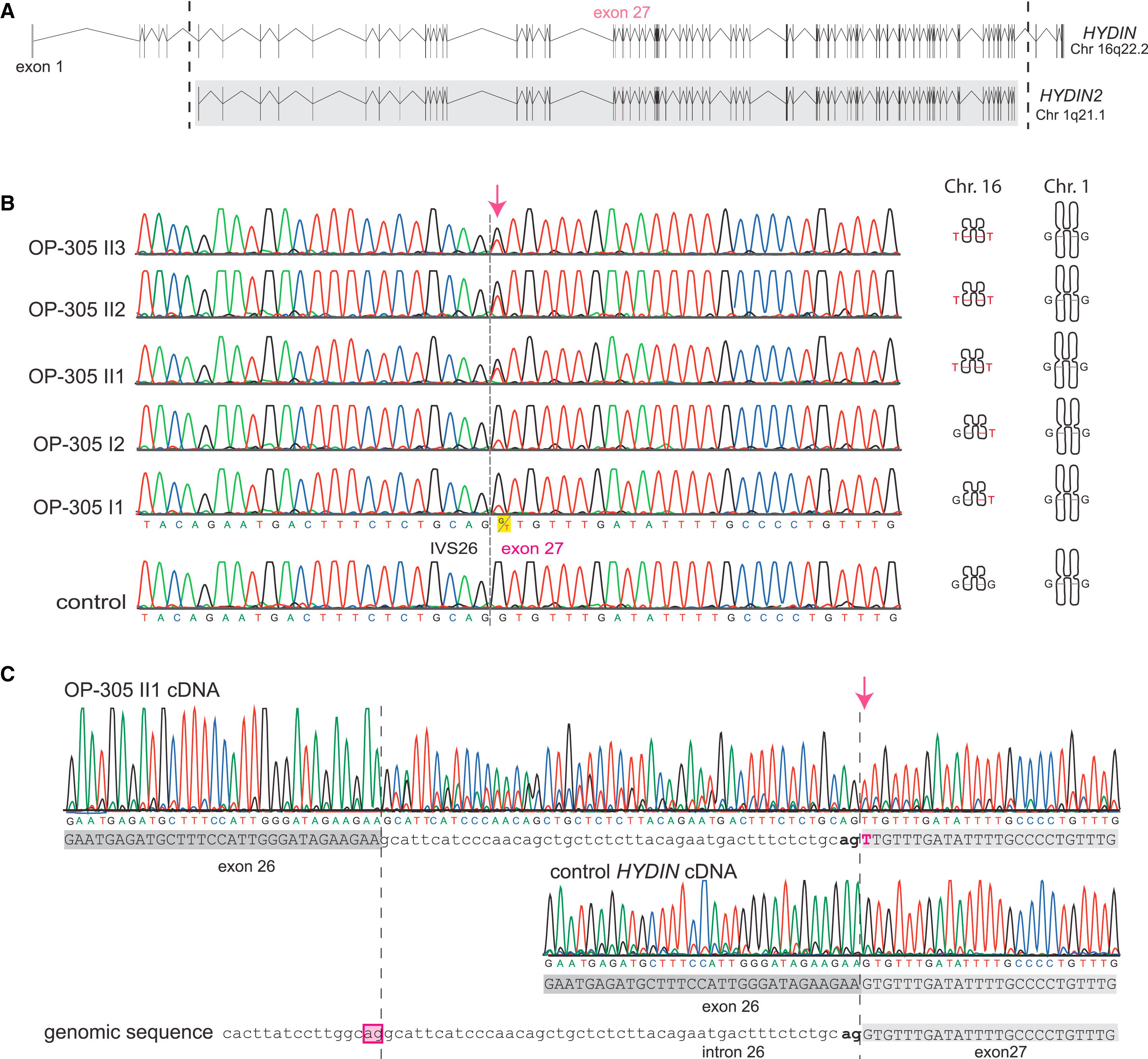
Figure 2: Identification of Recessive HYDIN Mutations in OP-305 and Verification that the c.3985G>T Splicing Mutation Causes Aberrant Splicing
(A) The ancestral duplication of the HYDIN locus from 16q22.2 to HYDIN2 (1q21.1) includes major parts of HYDIN (exons 6–83). The borders of the duplicated region are indicated by the dotted lines, and the gray box defines the paralogous genomic regions between the two loci HYDIN and HYDIN2. Because the duplication occurred very recently during human evolution, even intronic sequences are still conserved between HYDIN and HYDIN2. Thus, by PCR amplification of exon 27, four alleles are amplified, two alleles originate from HYDIN (chromosome 16), and two alleles originate from HYDIN2 (chromosome 1).</br>(B) Sequence chromatographs of exon 27 amplicons depict relative levels of the G>T DNA variant (c.3985G>T) in individuals from OP-305 at the first position of the conserved splice acceptor site of exon 27. Segregation analysis confirmed that this DNA variant, like SNP markers of the HYDIN locus (Figure 1), cosegregates with disease status. In contrast, SNP markers of the HYDIN2 locus do not show linkage (Figure 1). Thus, the segregation analysis indicates that the detected splicing mutation is located in HYDIN and not in the paralogous HYDIN2.</br>(C) To provide further evidence that the splicing variant is located within HYDIN, we performed HYDIN-specific RT-PCR analysis on individual OP-305 II1 and control respiratory epithelial cells (Figure S1 and Table S4). Identified in the sample from OP-305 II1 were exclusively abnormal transcripts resulting from malfunction of the HYDIN exon 27 splice acceptor site. The transcripts (upper panel) show a T nucleotide at c.3985 (arrow) at the first base of exon 27, and contained between the exon 26 and exon 27 boundary is an aberrant 47 bp insertion comprising the 3′ most end of IVS26. A control cDNA sequence (middle panel) is shown for comparison with the homozygous reference G nucleotide at c.3985 and normal exon 26/exon 27 splicing. The reference genomic sequence is shown in the bottom panel. The aberrant insertion can be explained by utilization of an upstream cryptic IVS26 splice acceptor (boxed) compared to the normal exon 27 splice acceptor (bold).
Licensed under: https://creativecommons.org/licenses/by/3.0/
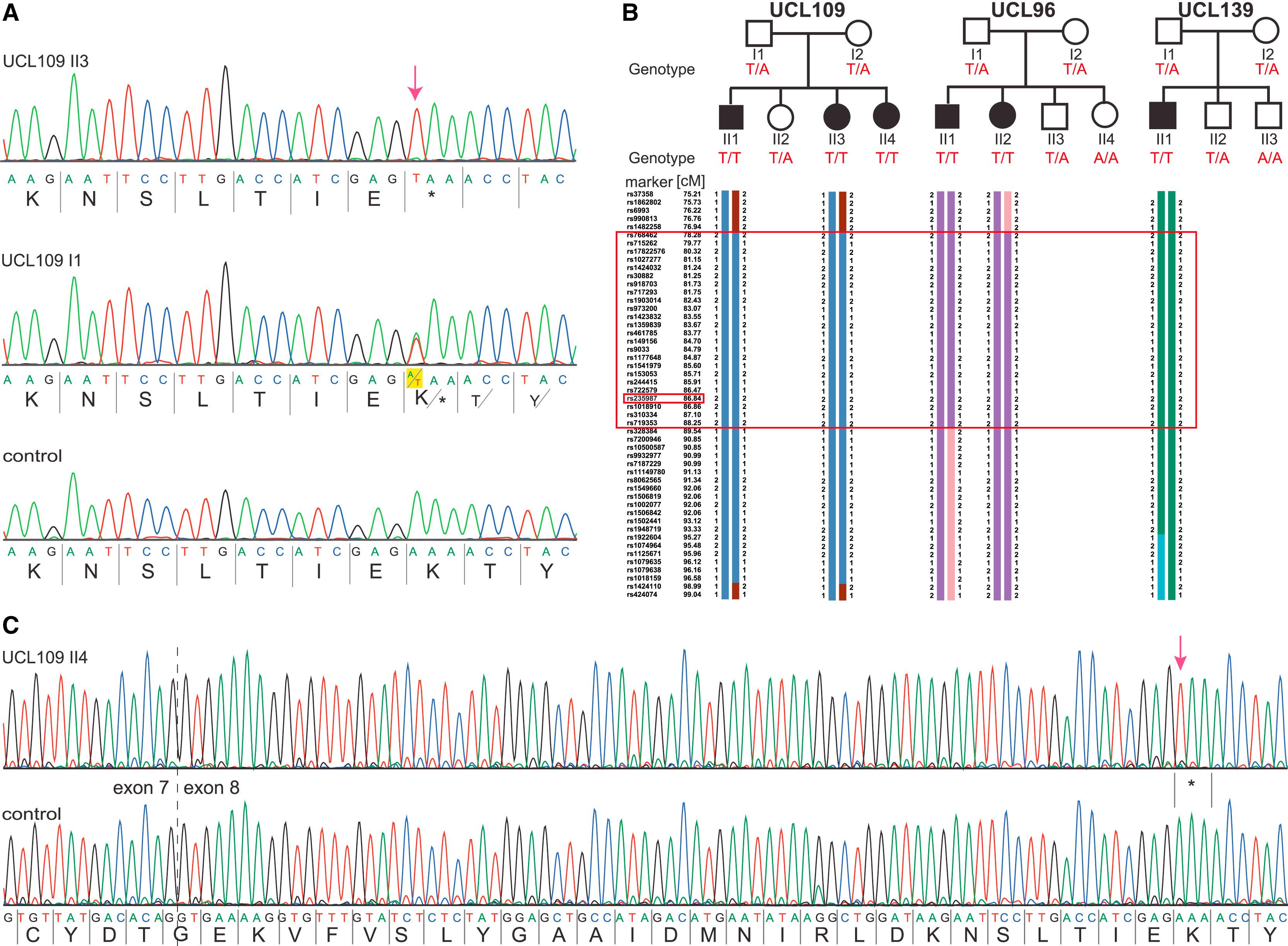
Figure 3: Identification of a Common HYDIN Mutation, c. 922A>T, in Faroe Island Families
(A) Sequence chromatographs of HYDIN exon 8 amplicons were generated with chromosome 16 HYDIN-specific primers (amplification-refractory mutation system [ARMS] method) and demonstrate homozygous A>T mutations (c.922A>T) predicted to create a homozygous AAA to TAA codon change, p.Lys307∗ (red arrow; amino acids are indicated below nucleotide sequence). Affected child UCL109 II3 (upper panel), homozygous for p.Lys307∗, and his father I1, a heterozygous carrier (middle), are compared to a control (bottom panel).</br>(B) Segregation-analysis genotypes confirmed that this nonsense variant cosegregates with disease status, which is inherited homozygously in all six affected children (T/T, black symbols), whereas all the parents and five unaffected siblings (white symbols) are carriers (T/A) or normal (A/A). Disease-associated haplotype reconstruction for the linkage region on chromosome 16 shows the flanking markers rs973200 and rs328384 spanning a 6.5 cM (8.8 Mb) region presumed to be a shared ancestral Faroe Island founder haplotype (large box). The location of HYDIN is indicated in the small box because marker rs235987 is intragenic.</br>(C) RT-PCR analysis of individual UCL109 II4 and control samples verified that the c.922A>T nonsense variant is contained within HYDIN on chromosome 16. All transcripts from the affected person (upper panel) showed the homozygous nonsense mutation (c.919A>T [p.Lys307∗]; red arrow); the HYDIN transcripts from a healthy control are shown in the lower sequence.
Licensed under: https://creativecommons.org/licenses/by/3.0/
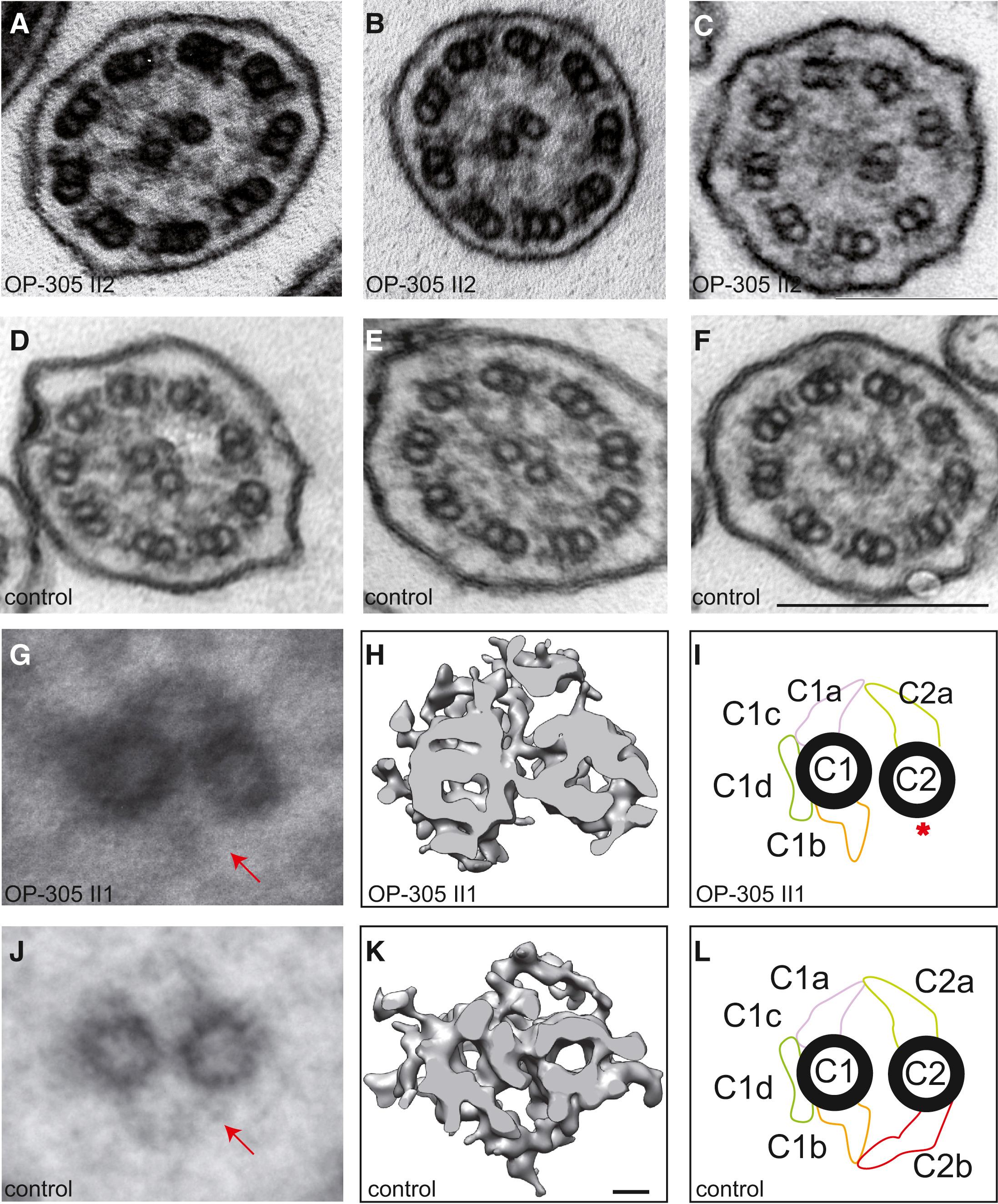
Figure 4: HYDIN Mutant Respiratory Ciliary Axonemes Show Defects of the CP Apparatus
Routine TEM cross-sections of HYDIN-mutant individual OP-305 II2 (A and B) exhibit in most cross-sections a normal 9+2 axonemal composition. When compared with control samples (D–F), the cross-sections of affected individuals rarely show 9+0 (not shown) or 8+1 (ciliary transposition defect in C) compositions. Please note that routine TEM cannot discern CP-apparatus projections. Image averages based on six affected (G) and six control (J) images aid visualization of the CP appendages. Note that the C2b projection is missing (marked with arrow) in the affected individuals. A detailed analysis of the CP apparatus by electron microscopic tomography (H and I) identified the absence of the C2b projection in HYDIN-mutant cilia from individual OP-305 II1 compared to control samples (K and L). Each rendered image (H and K) represents six cross-sections averaged from a dual-axis tomogram made up of 140 images at 2° tilts. The scale bars represent 10 nm (rendered picture) and 200 nm (TEM pictures).
Licensed under: https://creativecommons.org/licenses/by/3.0/
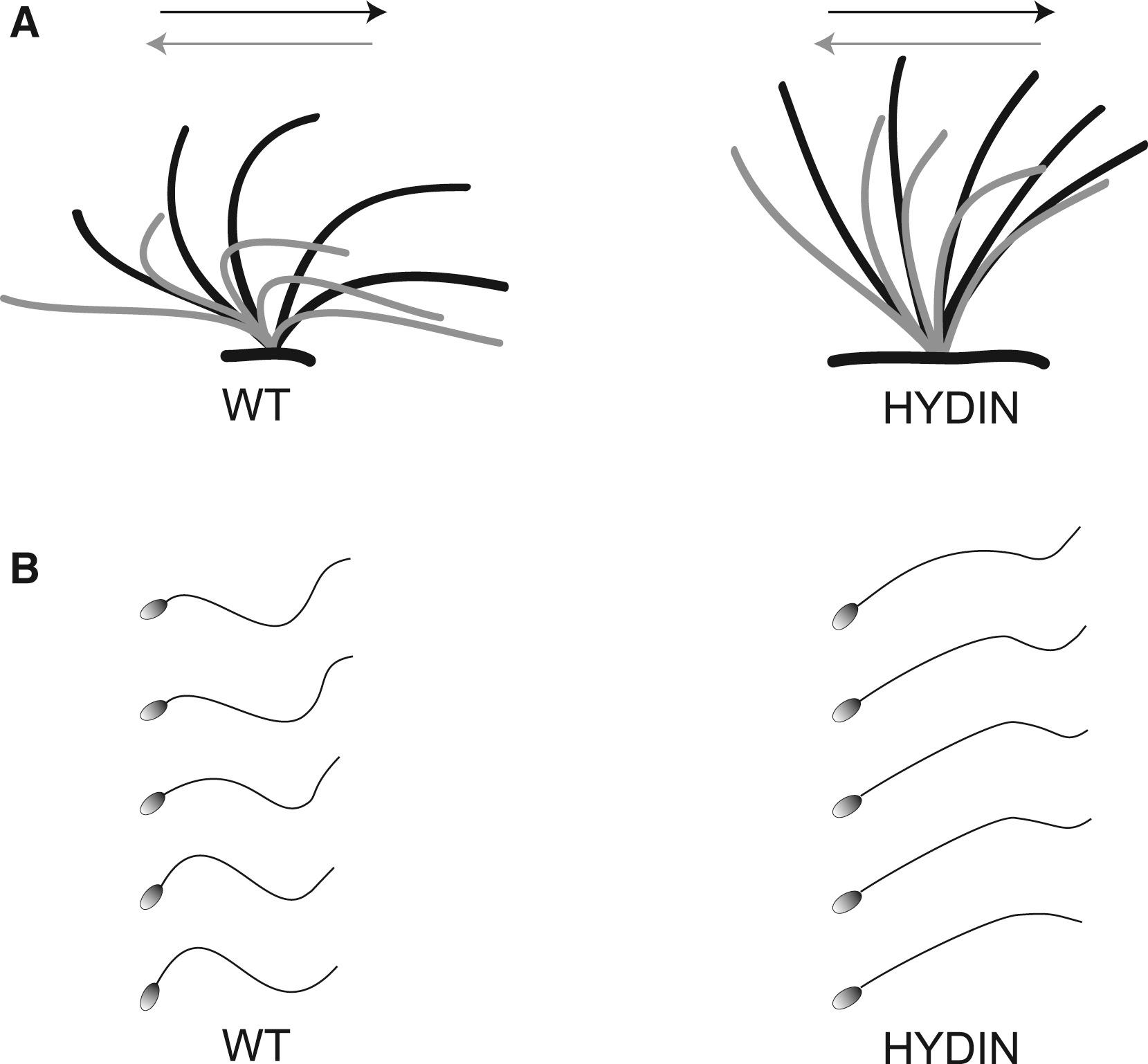
Figure 5: Functional Analysis of Respiratory Ciliary Beating and Sperm-Tail Movement
(A) Schematic of the effective stroke (black) and recovery stroke (gray) from wild-type respiratory cilia and an affected person of family OP-305 with HYDIN mutations.</br>(B) Schematic of sperm-tail movement of control and HYDIN-mutant sperm cells. Both HYDIN-mutant respiratory cilia and sperm tails exhibit a markedly reduced bending capacity. Note that most of the HYDIN-mutant sperm cells are immotile, and only a few show some residual motility. The bending of the proximal sperm flagellum and respiratory cilium appears to be more affected than distal bending.
Licensed under: https://creativecommons.org/licenses/by/3.0/
