Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility
Asaf Ta-Shma, Rim Hjeij, Zeev Perles, Gerard W. Dougherty, Ibrahim Abu Zahira, Stef J. F. Letteboer, Dinu Antony, Alaa Darwish, Dorus A. Mans, Sabrina Spittler, Christine Edelbusch, Sandra Cindrić, Tabea Nöthe-Menchen, Heike Olbrich, Friederike Stuhlmann, Isabella Aprea, Petra Pennekamp, Niki T. Loges, Oded Breuer, Avraham Shaag, Azaria J. J. T. Rein, Elif Yilmaz Gulec, Alper Gezdirici, Revital Abitbul, Nael Elias, Israel Amirav, Miriam Schmidts, Ronald Roepman, Orly Elpeleg, Heymut Omran, 27.08.2018
Abstract
The clinical spectrum of ciliopathies affecting motile cilia spans impaired mucociliary clearance in the respiratory system, laterality defects including heart malformations, infertility and hydrocephalus. Using linkage analysis and whole exome sequencing, we identified two recessive loss-of-function MNS1 mutations in five individuals from four consanguineous families: 1) a homozygous nonsense mutation p.Arg242* in four males with laterality defects and infertility and 2) a homozygous nonsense mutation p.Gln203* in one female with laterality defects and recurrent respiratory infections additionally carrying homozygous mutations in DNAH5. Consistent with the laterality defects observed in these individuals, we found Mns1 to be expressed in mouse embryonic ventral node. Immunofluorescence analysis further revealed that MNS1 localizes to the axonemes of respiratory cilia as well as sperm flagella in human. In-depth ultrastructural analyses confirmed a subtle outer dynein arm (ODA) defect in the axonemes of respiratory epithelial cells resembling findings reported in Mns1-deficient mice. Ultrastructural analyses in the female carrying combined mutations in MNS1 and DNAH5 indicated a role for MNS1 in the process of ODA docking (ODA-DC) in the distal respiratory axonemes. Furthermore, co-immunoprecipitation and yeast two hybrid analyses demonstrated that MNS1 dimerizes and interacts with the ODA docking complex component CCDC114. Overall, we demonstrate that MNS1 deficiency in humans causes laterality defects (situs inversus) and likely male infertility and that MNS1 plays a role in the ODA-DC assembly.
TA-SHMA, Asaf, et al. Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility. PLoS genetics, 2018, 14. Jg., Nr. 8, S. e1007602.
Publication: https://doi.org/10.1371/journal.pgen.1007602
 Disclaimer
Disclaimer
The publication Homozygous loss-of-function mutations in MNS1 cause laterality defects and likely male infertility by Asaf Ta-Shma, Rim Hjeij, Zeev Perles, Gerard W. Dougherty, Ibrahim Abu Zahira, Stef J. F. Letteboer, Dinu Antony, Alaa Darwish, Dorus A. Mans, Sabrina Spittler, Christine Edelbusch, Sandra Cindrić, Tabea Nöthe-Menchen, Heike Olbrich, Friederike Stuhlmann, Isabella Aprea, Petra Pennekamp, Niki T. Loges, Oded Breuer, Avraham Shaag, Azaria J. J. T. Rein, Elif Yilmaz Gulec, Alper Gezdirici, Revital Abitbul, Nael Elias, Israel Amirav, Miriam Schmidts, Ronald Roepman, Orly Elpeleg, Heymut Omran is published under an open access license: https://creativecommons.org/licenses/by/4.0/. Granted rights: share — copy and redistribute the material in any medium or format and adapt — remix, transform, and build upon the material for any purpose, even commercially.
Curation by the MFGA team Relevant data sets presented in the publication have been identified. If possible, annotations (title, general information, conditions, processed tissue types and processed cell types) have been added based on information from the publication. Data tables and images that provide a good overview on the publication's findings on the data set have been extracted from the publication and/or supplement. If not stated otherwise, images are depicted with title and description exactly as in the publication. Tables have been adjusted to the MFGA table format. Conducted adjustments are explained in the detailed view of the tables. However, titles and descriptions have been adopted from the publication.
Data set 1:
Other: Functional Study
Species
| Species |
|---|
| Human |
Conditions
| Human phenotype ontology | Participants | Comment |
|---|---|---|
| HP:0012864: Abnormal sperm morphology |
Cell Types
| Cell ontology | Maturity | Description | Species | Replicates | Cells per replicate |
|---|---|---|---|---|---|
| CL_0000066: epithelial cell | Adult | Respiratory epithelial cells | Human | ||
| CL_0000019: sperm | Adult | Human |
Images
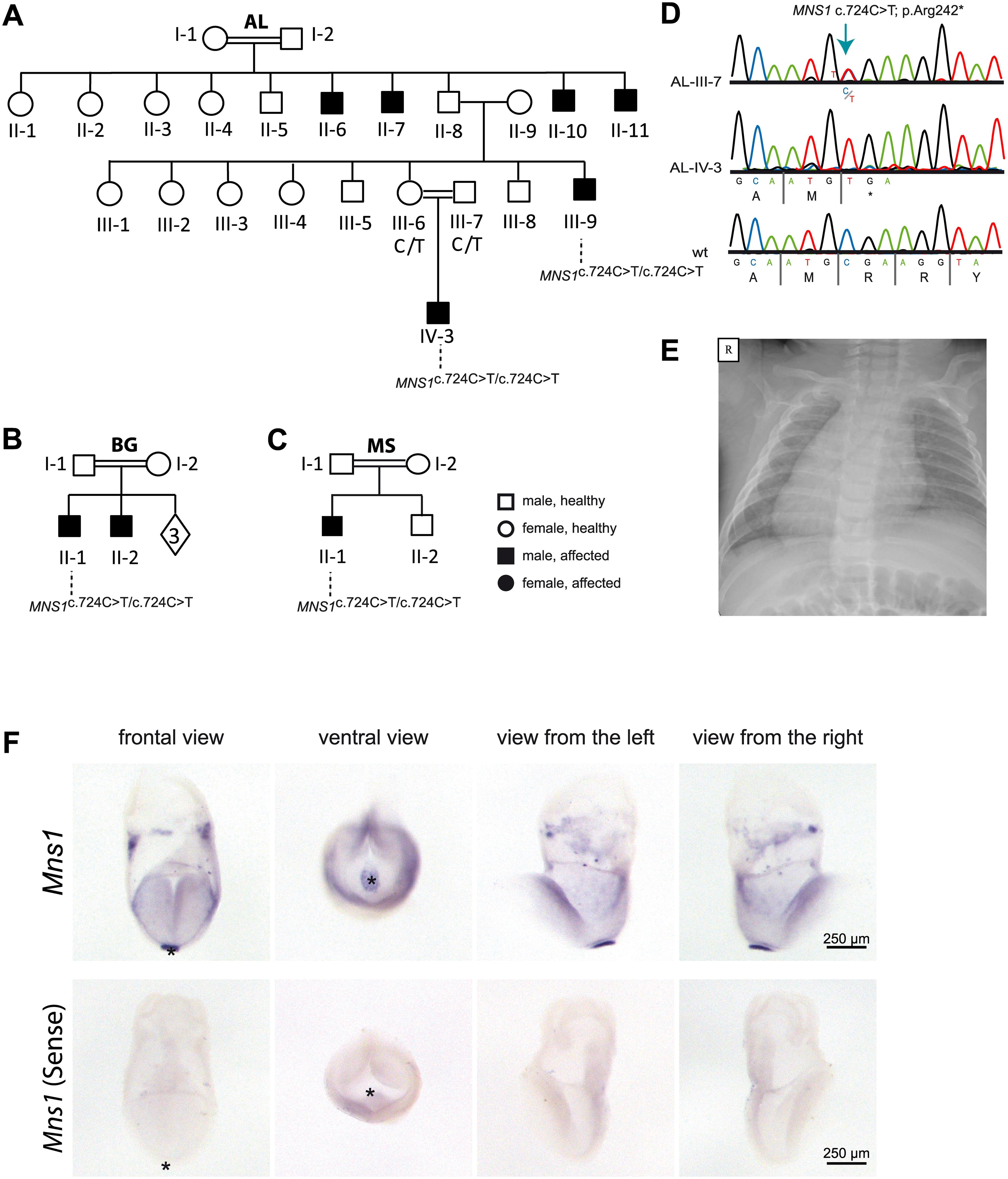
Figure 1: Homozygous loss-of-function mutations in MNS1 in three consanguineous families with laterality defects.
(A-C). Pedigrees of family AL with six affected individuals: AL-II-6, AL-II-7, AL-II-10, AL-II-11, AL-III-9 and AL-IV-3; family BG with two affected individuals: BG-II-1, BG-II-2 and family MS with one affected individual: MS-II-1. In total, 4 males (AL-III-9, AL-IV-3, BG-II-1 ad MS-II-1) carry homozygous loss-of-function MNS1 mutations (c.724G>A; p.Arg242*). The other affected individuals were not subject to DNA mutation analysis. The parents AL-III-6 and AL-III-7 of the affected individual AL-IV-3, the parents BG-I-1 and BG-I-2 of the affected individual BG-II-1 and the parents MS-I-1 and MS-I-2 of the affected individual MS-II-1 are both carriers of the mutations in a heterozygous state, confirming homozygosity by descent. (D) Bi-allelic MNS1 nonsense mutations (c.724T>C; p.Arg242*) in a carrier (AL-III-7), an affected individual (AL-IV-3), and a healthy control. (E) Chest X-ray of individual MS-II-1 showing situs inversus totalis. R.right. (F) In situ hybridization of wildtype mouse embryos detecting Mns1 expression at the ventral node. In wildtype mouse embryos at 8.25 dpc Mns1 is expressed predominantly at the ventral node as indicated by the strong blue signal (upper panel). Negative control experiments using the sense probe do not show any specific signal (lower panel). Asterisks in frontal and ventral view mark the position of the ventral node.
Licensed under: https://creativecommons.org/licenses/by/4.0/
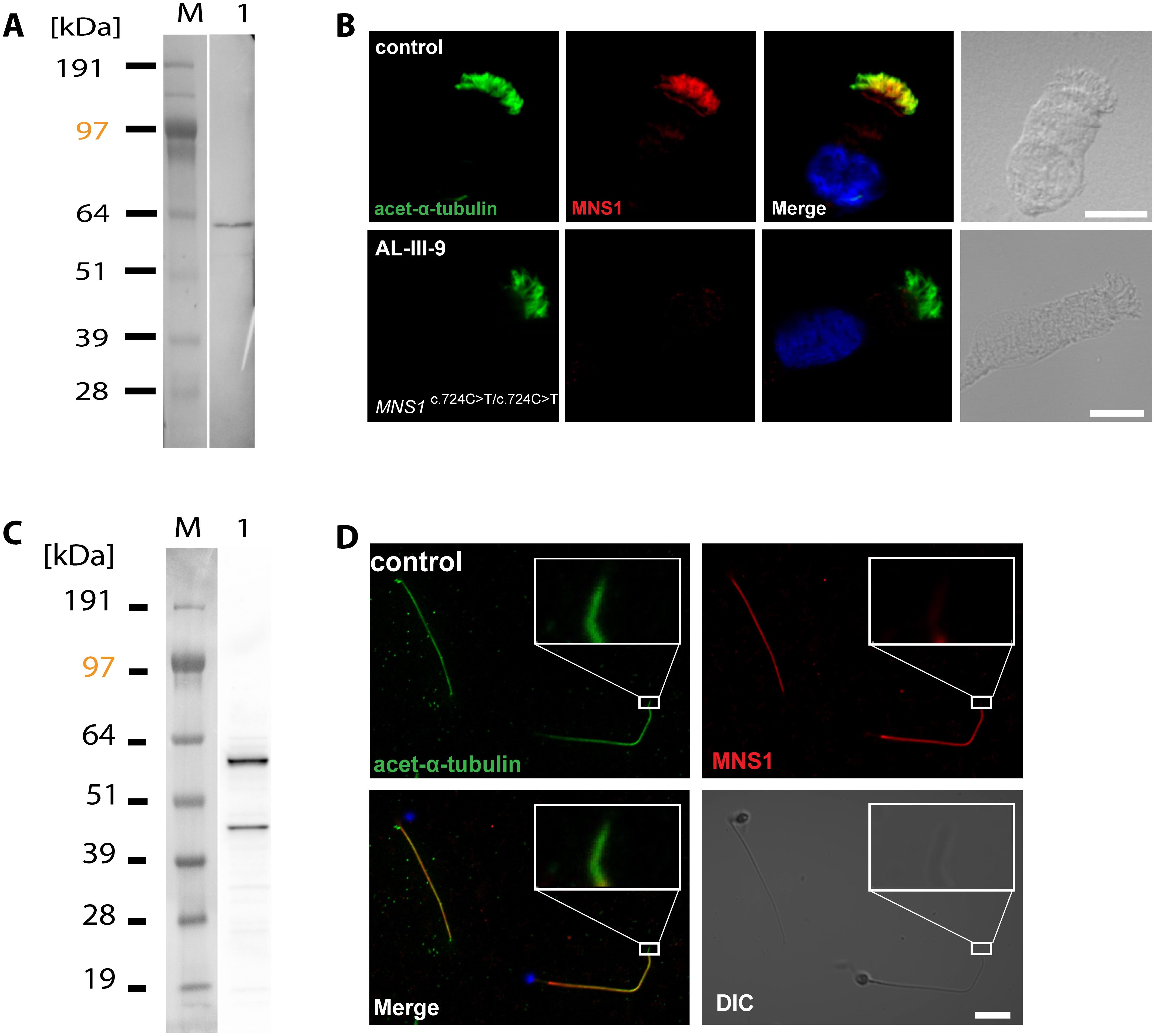
Figure 2: MNS1 localizes to human respiratory cilia and human sperm flagella.
(A) Western blot analysis of protein lysates from human respiratory cells (M, protein standard). MNS1 antibodies specifically detect a single band with the predicted size (~61kDa, lane 1). (B) Respiratory epithelial cells from control and affected individual AL-III-9 carrying bi-allelic MNS1 mutations. Cells were double-labeled with antibodies directed against acetylated alpha-tubulin (green) and MNS1 (red). Nuclei were stained with Hoechst 33342 (blue). Both proteins co-localize (yellow) along the ciliary axonemes in cells from the unaffected controls, while in respiratory cells of AL-III-9, MNS1 is undetectable in the ciliary axonemes, consistent with recessive loss-of-function MNS1 nonsense mutations. Scale bars, 10μm. (C) Western blot analysis of lysate from human whole sperm cells (M, protein standard). Anti-MNS1 antibody specifically detects a band at the predicted size (~61kDa, lane 1) and a band of approximately ~45kDa, indicating an isoform of MNS1 in sperm. (D) In human control spermatozoa, MNS1 (red) co-localizes with acetylated alpha-tubulin (green) along flagellar axonemes except at the endpiece (white box).
Licensed under: https://creativecommons.org/licenses/by/4.0/
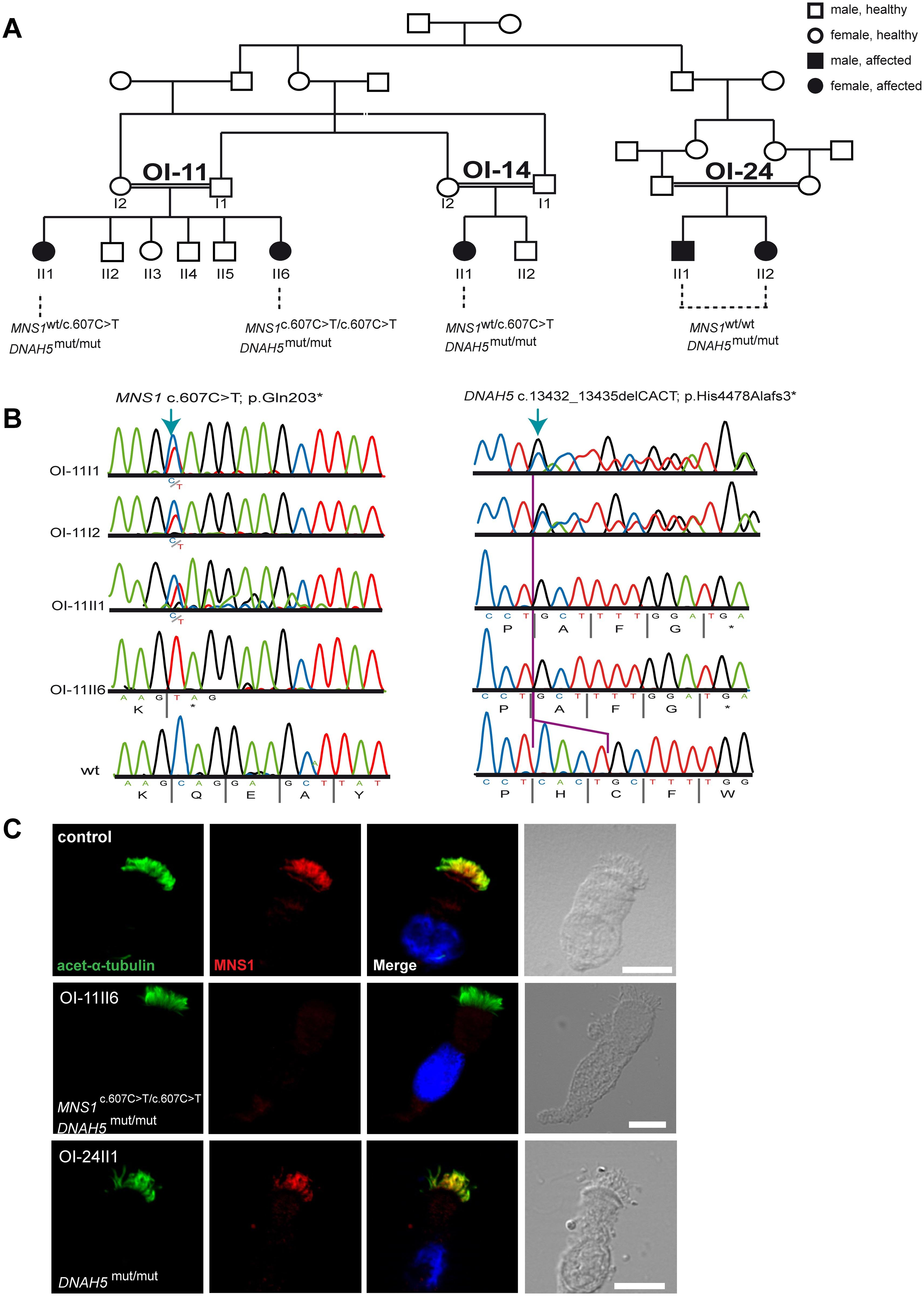
Figure 3: Identification of MNS1 loss-of-function mutations in a PCD-affected individual with DNAH5 mutations.
(A) Pedigree of families OI-11, OI-14 and OI-24: consanguinity of first degree. In total, five PCD-affected individuals carry homozygous mutations in DNAH5 (annotated as DNAH5(mut/mut)) of whom only one (OI-11 II6) carries additional homozygous mutations in MNS1 (c.607C>T; p.Gln203*). (B) Bi-allelic MNS1 nonsense mutations in OI-11 II6 (c.607C>T) predicting a premature termination of translation (p.Gln203*). The affected sibling OI-11 II1 and both parents OI-11 I1 and I2 are carriers of the mutant allele. Right panel. Bi-allelic DNAH5 nonsense mutations in OI-11 II6 and OI-11 II1 (c. c.13432_13435delCACT) predicting a premature termination of translation (p.His4478Alafs3*). Both parents OI-11 I1 and I2 are carriers of the mutant allele. (C) Respiratory epithelial cells from control and affected individuals: OI-11 II6 carrying bi-allelic MNS1 and DNAH5 mutations, and OI-24 II1 carrying the identical bi-allelic DNAH5 mutations as OI-11 II6. For space issues, OI-24 II1 is referred to in this and other Figures as DNAH5(mut/mut) instead of DNAH5(c.13432_13435delCACT/ c.13432_13435delCACT). Cells were double-labeled with antibodies directed against acetylated alpha-tubulin (green) and MNS1 (red). Nuclei were stained with Hoechst 33342 (blue). Both proteins co-localize (yellow) along the ciliary axonemes in cells from the unaffected controls and OI-24 II1, while in respiratory cells of OI-11 II6, MNS1 is undetectable in the ciliary axonemes, consistent with recessive loss-of-function MNS1 nonsense mutations. Scale bars, 10μm.
Licensed under: https://creativecommons.org/licenses/by/4.0/
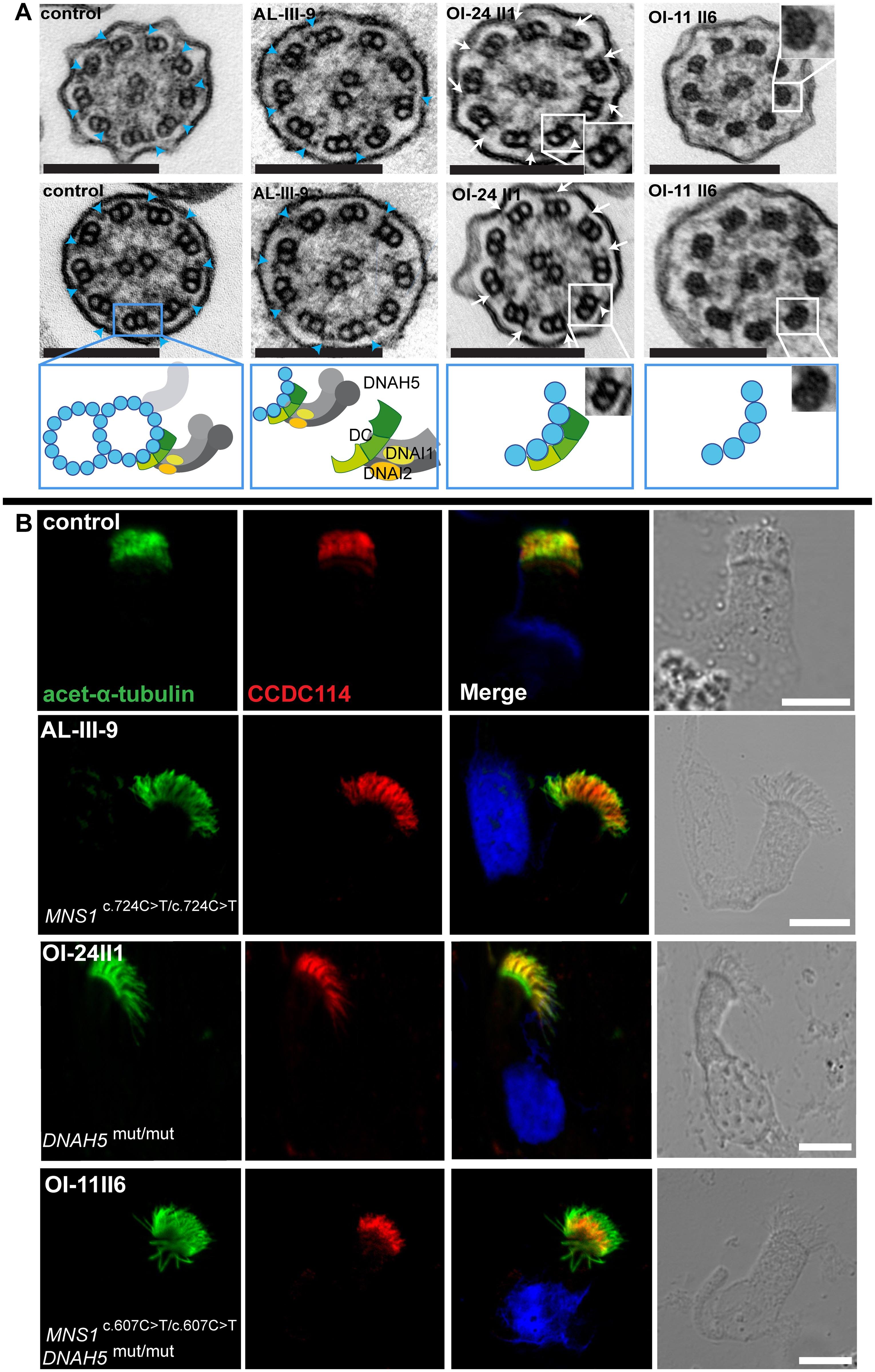
Figure 4: Mutations in MNS1 when combined with mutations in DNAH5 might result in defects of the ODA-microtubule docking complex in human respiratory epithelial cells.
(A) Transmission electron micrographs show subtle ultrastructural defects in affected individual AL-III-9 carrying bi-allelic MNS1 mutations with the occasional absence of only few ODAs (2–4 out of 9) in about half of the cross-sections (compared to control samples where all analyzed sections show an average of 8.7 ODAs, 9 analyzed sections from the MNS1-deficient ciliary axonemes show an average of 6 ODAs). However, TEM show complete absence of ODAs in PCD-affected individuals OI-24 II1 (DNAH5 mutations) and OI-11 II6 (MNS1 and DNAH5 mutations) compared to a control without PCD. In the healthy control, outer dynein arms are visible (blue arrows). However, the cilia from OI-24 II1 still have the ODA-DC (small projections marked by white arrows) whereas the cilia from OI-11 II6 do not, suggesting that MNS1 deficiency when combined with DNAH5 deficiency might cause defects in ODA-DC assembly. Below the control TEM section a schematic illustrating a microtubular doublet with attached ODA docking complex (ODA-DC) and the double-headed ODA complex proteins with dynein heavy chain DNAH5 and dynein intermediate chains DNAI1 and DNAI2. In affected individual AL-III-9, a partial defect is observed; in OI-24II1, a schematic where the ODA complex is absent while the ODA-DC is still retained; in OI-11II6, a schematic where both ODA and ODA docking complexes are absent. Scale bars, 0.1 μm. (B) Respiratory epithelial cells from control and affected individuals: AL-III-9 carrying bi-allelic MNS1 mutations, OI-11 II6 carrying bi-allelic MNS1 and DNAH5 mutations and OI-24 II1 carrying no mutations in MNS1 but identical bi-allelic DNAH5 mutations as OI-11 II6. For space issues, OI-24 II1 is described as DNAH5(mut/mut) instead of DNAH5(c.13432_13435delCACT/ c.13432_13435delCACT). Cells were double-labeled with antibodies directed against acetylated alpha-tubulin (green) and CCDC114 (HPA042524, Atlas antibodies) (red). Nuclei were stained with Hoechst 33342 (blue). Both proteins co-localize (yellow) along the ciliary axonemes in cells from the unaffected controls, AL-III-9 and OI-24 II1, while in cells of OI-11 II6, CCDC114 localizes only to the proximal part of the ciliary axonemes, indicating that recessive loss-of-function mutations in MNS1 when combined with loss-of-function mutations in DNAH5 might affect the distal localization of ODA-DC associated proteins and might play a role in docking or anchoring the ODA subunits or in regulating this process. Scale bars, 10μm.
Licensed under: https://creativecommons.org/licenses/by/4.0/
Results
- Table 2: Clinical features of the affected individuals first
- Table S3: List of homozygous variants left after filtering in individual BG-II-1 first
- Table S4: Homozygous variants after filtering in individual MS-II-1 first
- Table S2: List of homozygous variants left after filtering in individual AL-IV-3 first
- Table 1: Overview of the study cohorts analyzed by whole exome sequencing first
